Chemical and Biological Investigations of Vietnamese ... · Lyngbya majuscula in Khanh Hoa province...
Transcript of Chemical and Biological Investigations of Vietnamese ... · Lyngbya majuscula in Khanh Hoa province...

Ernst Moritz Arndt-Universität Greifswald Mathematisch-Naturwissenschaftliche Fakultät
Chemical and Biological
Investigations of Vietnamese
Cyanobacteria
Inauguraldissertation
zur
Erlangung des akademischen Grades
doctor rerum naturalium (Dr. rer. nat.)
an der Mathematisch-Naturwissenschaftlichen Fakultät
der
Ernst-Moritz-Arndt-Universität Greifswald
vorgelegt von Le Thi Anh Tuyet
geboren am 19.05.1973 in Thanh Hoa, Vietnam
Greifswald, Juli 2010

Dekan: Prof. Dr. Klaus Fesser………………………………………………
1. Gutachter:
Prof. Dr. Johannes F. Imhoff
2. Gutachter:
PD. Dr. Sabine Mundt
Tag der
Promotion:……16.09.2010……………………………………………………………

Erklärung
Hiermit erkläre ich, daß diese Arbeit bisher von mir weder an der
Mathematisch-Naturwissenschaftlichen Fakultät der Ernst-Moritz-Arndt-Universität
Greifswald noch einer anderen wissenschaftlichen Einrichtung zum Zwecke der
Promotion eingereicht wurde.
Ferner erkläre ich, daß ich diese Arbeit selbständig verfaßt und keine anderen
als die darin angegebenen Hilfsmittel benutzt habe.
Greifswald, den 19 Juli 2007 Unterschrift
Le Thi Anh Tuyet

Table of Contents
1 INTRODUCTION.....................................................................................................1
1.1 Cyanobacteria.................................................................................................................. 1 1.1.1 Cyanobacterial physiology and morphology ..................................................................... 1 1.1.2 Ecology of cyanobacteria................................................................................................ 2 1.1.3 Classification of cyanobacteria........................................................................................ 3
1.2 Cyanobacteria–a new and rich source of novel bioactive compounds with pharmaceutical potential ........................................................................................................ 6
1.2.1 Antimicrobials .............................................................................................................. 9 1.2.2 Cytotoxic and antitumoural activities............................................................................. 12 1.2.3 Antiviral activity.......................................................................................................... 18 1.2.4 Toxins and other pharmacologically active compounds ................................................... 19
1.3 Aim of the work.............................................................................................................. 22
2 MATERIALS AND METHODS ...........................................................................23
2.1 Biological materials....................................................................................................... 23 2.1.1 Soil cyanobacteria........................................................................................................ 23 2.1.2 Marine cyanobacteria ................................................................................................... 29 2.1.3 Bacteria, yeast, and cancer cell lines as test organisms .................................................... 31
2.2 Chemicals....................................................................................................................... 31 2.2.1 Cultivation of cyanobacteria ......................................................................................... 31 2.2.2 Cultivation of bacteria and yeast as test organisms .......................................................... 32 2.2.3 General laboratory chemicals.......................................................................................... 32 2.2.4 Chemical reagents........................................................................................................ 32 2.2.5 Fatty acid analysis ......................................................................................................... 33
2.3 Solvents............................................................................................................................ 33
2.4 Equipment in generally company, town, country ...................................................... 34 2.4.1 Cultivation of cyanobacteria ......................................................................................... 34 2.4.2 Extraction ..................................................................................................................... 34 2.4.3 Isolation of secondary metabolites................................................................................. 35
2.4.3.1 Thin layer chromatography (TLC).......................................................................... 35 2.4.3.2 Preparative TLC ................................................................................................... 35 2.4.3.3 Open column chromatography ............................................................................... 35 2.4.3.4 HPLC .................................................................................................................. 36
2.4.4 Agar plate diffusion test ................................................................................................. 36 2.4.5 Bioautographic TLC assay.............................................................................................. 37 2.4.6 Fatty acid analyses ....................................................................................................... 37
2. 5 Cultivation of cyanobacteria ....................................................................................... 38 2.5.1 The stock culture ......................................................................................................... 38 2.5.2 The batch culture ......................................................................................................... 38 2.5.3 The large scale culture.................................................................................................. 38
2.6 Extraction ....................................................................................................................... 39 2.6.1 Extraction of intracellular compounds............................................................................ 39 2.6.2 Extraction of extracellular compounds ........................................................................... 40

2.7 Bioassays....................................................................................................................... 41 2.7.1 Assays for antimicrobial activity ................................................................................... 41
2.7.1.1 Agar diffusion assay.............................................................................................. 41 2.7.1.2 Bioautographic TLC assay..................................................................................... 42
2.7. 2 Assays for cytotoxic activity ........................................................................................ 43
2.8 Fractionation and isolation of the secondary metabolites of 6 cyanobacterial strains................................................................................................................................................. 43
2.8.1 Fractionation and isolation of the secondary metabolites of Westiellopsis sp.VN ................. 43 2.8.2 Fractionation and isolation of the secondary metabolites of Calothrix javanica ................. 45 2.8.3. Fractionation and isolation of the secondary metabolites of Scytonema ocellatum .............. 47 2.8.4 Fractionation and isolation of the secondary metabolites of Anabaena sp.......................... 49 2.8.5 Fractionation and isolation of the secondary metabolites of Nostoc sp. ............................. 50 2.8.6 Fractionation and isolation of the secondary metabolites of Lyngbya majuscula .................. 51
2.8.6.1 Method 1.............................................................................................................. 51 2.8.6.2 Method 2.............................................................................................................. 53
2.9 Structure elucidation of the isolated secondary metabolites................................... 55 2.9.1 Structure elucidation of compounds isolated from Westiellopsis sp.VN and Lyngbya majuscula............................................................................................................................................. 55 2.9.2 Structure elucidation of compounds isolated from Calothrix javanica and Scytonema ocellatum .............................................................................................................................. 56 2.9.3 Structure elucidation of compounds isolated from Anabaena sp. ........................................ 56 2.9.4 Structure elucidation of compounds isolated from Nostoc sp. ............................................ 56
2.10 Investigation of the active ethyl acetate extract of Westiellopsis sp. VN growth medium................................................................................................................................... 56
2.11 Gas chromatography- mass spectrometry ............................................................... 57
2.12 Culture optimization of Westiellopsis sp.VN............................................................ 59 2.12.1 The effects of nitrogen deficiency................................................................................ 59 2.12.2 The effects of cultivation time (culture age).................................................................. 59
3 RESULTS ..............................................................................................................61
3.1 Screening of antibacterial activity ............................................................................... 61 3.1. 1 Extract preparation ...................................................................................................... 61 3.1.2 Screening of crude extracts ........................................................................................... 61
3.2 Chemical investigation and culture optimization of Westiellopsis sp. VN................ 65 3.2.1 Chemical investigation of methanol extract obtained from biomass .................................. 65
3.2.1.1 Fractionation of methanol extract by silica gel column chromatography .................... 65 3.2.1.2 Seperation of the combined fractions (FI, FII, and FIII) by sephadex LH-20 column .... 66 3.2.1.3 Purification of fraction WF1 using reversed-phase HPLC .......................................... 67 3.2.1.4 Structure elucidation of isolated compounds of Westiellopsis sp.VN ......................... 68
3.2.1.4.1 Structure elucidation of fraction WF1-3 (compound 1) ....................................... 68 3.2.1.4.2 Structure elucidation of fraction WF1-5............................................................. 69 3.2.1.4.3 Structure elucidation of fraction WF1-6 (compound 4)........................................ 73 3.2.1.4.4 Identification of compounds in fraction WF1-8 .................................................. 74
3.2.2 Chemical investigation of the active ethyl acetate extract resulting from cultivation medium of Westiellopsis sp. VN .............................................................................................................. 76 3.2.3 Culture optimization of Westiellopsis sp.VN .................................................................. 79
3.2.3.1 Nitrogen deficiency............................................................................................... 79 3.2.3.2 The effect of incubation time on biomass production and antibacterial production........ 81
3.3 Chemical investigation of Calothrix javanica ............................................................ 82 3.3.1 Fractionation of methanol extract from biomass by RPC18 chromatography ........................ 82 3.3.2 Purification of fraction CJFII by semi-preparative reversed-phase HPLC .......................... 83

3.3.3 Structure elucidation of fraction CJFII-4 (compound 7)....................................................... 85
3.4 Chemical investigation of Scytonema ocellatum...................................................... 90 3.4.1 Fractionation of methanol extract obtained from biomass by RP C18 column chromatography............................................................................................................................................. 90 3.4.2 Separation of fraction SOFII using silica gel column ....................................................... 91 3.4.3 Purification of the pooled fractions using reversed-phase RP HPLC ................................. 91 3.4.4 Structure elucidation of fraction FSO3 ........................................................................... 93
3.5 Chemical investigation of Anabaena sp. .................................................................... 94 3.5.1 Purification of EtOAc extract using semi-preparative reversed-phase HPLC ..................... 94 3.5.2 Structure elucidation of fraction AF6 (compound 8) ........................................................ 96
3. 6 Chemical investigation of Nostoc sp. ........................................................................ 98 3.6.1 Fractionation of methanol extract obtained from biomass using silica gel column.............. 98 3.6.2 Separation of the acitve fraction NFIV using RP C18 column chromatography .................... 98 3.6.3 Purification of the active fraction NFIV-1 using semi-preparative reversed-phase HPLC ........ 99 3.6.4 Structure elucidation of fraction NsF2 .......................................................................... 100
3.7 Chemical investigation of Lyngbya majuscula ........................................................ 100 3.7.1 Isolation of cytotoxic compounds of methanol extract obtained from biomass according to method 1 ............................................................................................................................. 101
3.7.1.1 Fractionation of methanol extract by silica gel column chromatography .................. 101 3.7.1.2 Separation of fraction F8 by silica gel column chromatography ............................... 102 3.7.1.3 Purification of fraction F8-3 by preparative TLC ..................................................... 103 3.7.1.4 Structure elucidation of fraction F8-3-2 (compound 9).............................................. 103
3.7.2 Isolation of cytotoxic compounds of methanol extract obtained from biomass according to method 2 ............................................................................................................................. 105
3.7.2.1 Separation of methanol extract by silica gel column chromatography ...................... 105 3.7.2.2 Purification of fraction F10 using semi-preparative reversed-phase HPLC ................ 107 3.7.2.3 Structure elucidation of fractions F10-3 and F10-5 ..................................................... 108
3.7.2.3.1 Structure elucidation of fraction F10-3 (compound 10)...................................... 108 3.7.2.3.2 Structure elucidation of fraction F10-5 (compound 11) ........................................ 110
3.7.3 Fatty acid analysis...................................................................................................... 112
4 DISCUSSION ......................................................................................................113
4.1 Screening of crude extracts for antibacterial activity ............................................. 113 4.1.1 Selection of antibiotic screening and cyanobacterial strains ........................................... 113 4.1.2 Cultivation and extraction........................................................................................... 115 4.1.3 Antibacterial activity.................................................................................................... 116
4.2 Chemical investigation and culture optimization of Westiellopsis sp. VN............ 117 4.2.1 Selection of Westiellopsis sp.VN ................................................................................. 117 4.2.2 Active intracellular metabolites of Westiellopsis sp.VN strain.......................................... 118 4.2.3 Chemical composition of volatile extracellular compounds of Westiellopsis sp.VN strain .. 122 4.2.4 Cultivation optimization of Westiellopsis sp.VN strain.................................................... 123 4.2.5 Effect of incubation time on biomass production and antimicrobial compound accumulation of Westiellopsis sp.VN strain ................................................................................................ 128
4.3 Chemical investigation of Calothrix javanica and Scytonema ocellatum ............... 130 4.3.1 Selection of Calothrix javanica ................................................................................... 130 4.3.2 Selection of Scytonema ocellatum ................................................................................. 130 4.3.3 New cyclic peptide of Calothrix javanica and Scytonema ocellatum............................... 130
4.4 Chemical investigation of Anabaena sp. .................................................................... 136 4.4.1 Selection of Anabaena sp............................................................................................ 136 4.4.2 Active compound of Anabaena sp. .............................................................................. 137

4.5 Chemical investigation of Nostoc sp. ......................................................................... 138 4.5.1 Selection of Nostoc sp. ................................................................................................. 138 4.5.2 Active compounds of Nostoc sp. ................................................................................... 139
4.6 Chemical investigation of the marine Lyngbya majuscula ....................................... 139 4.6.1 Selection of the marine cyanobacterium L. majuscula collected in Vietnam .................... 139 4.6.2 Cytotoxic compounds of the marine cyanobacterium Lyngbya majuscula collected in Vietnam .............................................................................................................................. 140
4.7 Conclusion ................................................................................................................... 142
SUMMARY ..............................................................................................................143
REFERENCES.........................................................................................................143
ACKNOWLEDGMENTS .......................................................................................168
CURRICULUM VITAE..........................................................................................171
LIST OF PUBLICATIONS AND OTHER SCIENTIFIC ACHIEVEMENTS .173
APPENDIX ...............................................................................................................174

List of Figures Figure 1-1: Crytophycin 1 and crytophycin 8................................................................ 11
Figure 1-2: Norharmane from cyanobacteria................................................................. 12
Figure 1-3: Cryptophycin 1 and 52, potent antitumor agents from cyanobacteria ........ 14
Figure 1-4: Prominent anticancer marine cyanobacterial secondary metabolites and synthetic analogues ........................................................................................................... 15
Figure 1-5: Borophycin from cyanobacteria.................................................................. 17
Figure 1-6: Nostocarboline from Nostoc 78-12A.......................................................... 21
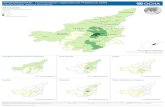
Figure 2-1: Map of Vietnam with the locality of 12 cyanobacterial strains in Dak Lak province ..................................................................................................................... 23
Figure 2-2: Morphology of 12 cyanobacterial strains ................................................... 28
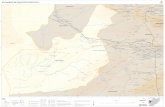
Figure 2-3: Map of Vietnam with the collection area and collection place of Lyngbya majuscula in Khanh Hoa province indicated ..................................................... 29
collection place of Lyngbya majuscula in Khanh Hoa province indicated ...................... 29
Figure 2-4: Natural habit of Lyngbya majuscula and microscopic view of filament of Lyngbya majuscula ....................................................................................................... 31
Figure 2-5: Agilent 6890N gas chromatograph and mass selective detector ............... 37
Figure 2-6: Cultivation of cyanobacteria ....................................................................... 39
Figure 3-1: Antibacterial activity of cyanobacterial extracts against the Gram positive bacterium Bacillus subtilis ATCC 6501.............................................................. 62
Figure 3-2: Antibacterial activity of cyanobacterial extracts against the Gram positive bacterium Staphylococcus aureus ATCC 6538 ................................................................ 62
Figure 3-3: Antibacterial activity of cyanobacterial extracts against the Gram negative bacterium Escherichia coli ATCC 11229 .......................................................... 63
Figure 3-4: Antibacterial activity of cyanobacterial extracts against the Gram negative bacterium Pseudomonas aeruginosa ATCC 22853 ........................................... 64
Figure 3-5: Semi-preparative RP HPLC chromatogram of WF1 .................................... 67
Figure 3-6: Ambiguine D isonitrile ............................................................................... 68
Figure 3-7: UV and ESI-MS of fraction WF1-3 ............................................................. 68
Figure 3-8: UV and ESI-MS of compounds of fraction WF1- 5..................................... 69
Figure 3-9: MS data of peak 1 of fraction WF1-5 ........................................................... 70
Figure 3-10: Ambiguine C isonitrile.............................................................................. 70
Figure 3-11: MS data of peak 2 of fraction WF1-5 ........................................................ 71
Figure 3-12: Fischerellin A............................................................................................ 71
Figure 3-13: MS data of peak 3 of fraction WF1-5 ........................................................ 72
Figure 3-14: Ambiguine B isonitrile.............................................................................. 73
Figure 3-15: UV and ESI-MS of fraction WF1-6 ........................................................... 73
Figure 3-16: ESI-MS of compounds of fraction WF1- 8 ................................................ 74
Figure 3-17: ESI-MS of peak 2 of fraction WF1- 8 ........................................................ 75
Figure 3-18: ESI-MS of peak 3 of fraction WF1- 8......................................................... 76
Figure 3-19: Bioautographic assay of ethyl acetate extract from cultivation medium of Westiellopsis sp.VN against S.aureu ............................................................................ 77
Figure 3-20: Analytical HPLC of MeOH fraction of EtOAc extract of cultivation medium of Westiellopsis sp. VN ...................................................................................... 77
Figure 3-21: Fermenters for cultivation of Westiellopsis sp.VN ................................ 79
Figure 3-22: Agar diffusion test of extracts prepared from biomass and cultivation medium of Westiellopsis sp.VN grown in BG-11 media ................................................. 80

Figure 3-23a: The effect of incubation time on dry weight and antibiotic production of Westiellopsis sp.VN...................................................................................................... 81
Figure 3-23b: Agar diffusion test of MeOH extracts of Westiellopsis sp.VN biomass ............................................................................................................................. 81
Figure 3-24: Analytical HPLC chromatogram of fraction CJFII .................................. 83
Figure 3-25: Analytical HPLC chromatogram of fraction CJFII-4 .................................. 84
Figure 3-26: Daklakapeptin .......................................................................................... 85
Figure 3-27: Semi-preparative RP HPLC chromatogram of the pooled fractions SOFII-5 and SOFII-6 ........................................................................................................... 92
Figure 3-28: Analytical HPLC chromatogram of fraction FSO3 ................................... 93
Figure 3-29: Semi-preparative RP HPLC chromatogram of the crude EtOAc extract from culture medium ........................................................................................................ 95
Figure 3-30: Agar diffusion test of 7 fractions obtained from ethyl acetate extract of culture medium of Anabaena sp. ..................................................................................... 96
Figure 3-31: Fluourensadiol........................................................................................... 96
Figure 3-32: Semi-preparative RP HPLC chromatogram of fraction NFIV-1 ................ 99
Figure 3-33: Thin layer chromatogram of 15 fractions obtained from silica gel column ............................................................................................................................ 102
Figure 3-34: 17-debromo-3, 4didehydro-3-deoxy –aplysiatoxin ................................ 103
Figure 3-35: ESI-MS spectrum of fraction F8-3-2 ........................................................ 104
Figure 3-36: Thin layer chromatogram of 22 fractions obtained from silica gel column ............................................................................................................................ 105
Figure 3-37: Semi-preparative RP HPLC chromatogram of F10 .................................. 107
Figure 3-38: Debromoaplysiatoxin............................................................................. 108
Fgure 3-39: UV and ESI-MS spectrum of compound 10 ............................................ 110
Figure 3-40: 3,4-didehydro-3-deoxy-aplysiatoxin....................................................... 110
Figure 3-41: UV and ESI-MS spectrum of compound 11........................................... 111
Figure 4-1: Fischerellins from cyanobacteria .............................................................. 121 Figure 4-2: Sequence of daklakapeptin ....................................................................... 131 Figure 4-3: Analytical HPLC chromatogram of methanol extract of Calothrix javanica .......................................................................................................................... 132
Figure 4-4: Analytical HPLC chromatogram of methanol extract of Scytonema ocellatum ........................................................................................................................ 133
Figure 4-5: The phylogenetic relationships of cyanobacteria inferred from 16S rRNA nucleotide sequence ........................................................................................................ 134

List of Tables Table 1-1: The orders of cyanobacteria according to the botanical classification and their correspondence to the subsections of the bacteriological code .................................. 4
Table 2-1: Cyanobacterial strains .................................................................................. 24
Table 2-2: Step gradient used in purification of fraction WF1 by semi-preparative HPLC ................................................................................................................................ 44
Table 2-3: Step gradient used in purification of fraction CJFII by semi-preparative HPLC ................................................................................................................................ 46
Table 2-4: Step gradient used in purification the pooled fractions (SOFII-5,SOFII-6) .... 48
Table 2-5: Step gradient used for purification of the EtOAc extract by semi-preparative HPLC ............................................................................................................. 49
Table 2-6: Step gradient used for purification of fraction NFIV-1 by semi-preparative HPLC ................................................................................................................................ 51
Table 2-7: Step gradient used in purification of fraction F10 by semi-preparative HPLC ................................................................................................................................ 54
Table 3-1: Dry weight of extracts from biomass (1g) and culture media (1L) of 12 cyanobacterial strains........................................................................................................ 61
Table 3-2: Fractionation of methanol extract from Westiellopsis sp. VN biomass by silica gel chromatography and antibacterial activity of fractions to S. aureus ................. 65
Table 3-3: Fractionation of combined fractions FI, FII, and FIII from Westiellopsis sp. VN biomass by LH-20 chromatography and antibacterial activity of fractions to S. aureus................................................................................................................................ 66
Table 3-4: Fractionation of WF1 from Westiellopsis sp. VN biomass by semi-preparative reversed-phase HPLC and antibacterial activity of fractions to S. aureus .... 67
Table 3-5: 1H NMR data of compound 1 compared with literature data of ambiguine
D isonitrile ........................................................................................................................ 69
Table 3- 6: 1H NMR data of compound 3 compared with literature data* of
fischerellin A..................................................................................................................... 72
Table 3-7: Comparison of 1H NMR data of compound 4 with reported data*.............. 74
Table 3-8: Compounds of MeOH fraction analyzed as methyl ester in n-hexane after hydrolysis /derivatization.................................................................................................. 78
Table 3-9: Compounds of MeOH fraction analyzed as methyl ester in MeOH after hydrolysis/derivatization................................................................................................... 78
Table 3-10: Dry biomass and antibacterial activity against S.aureus of extracts in BG-11 medium.................................................................................................................. 80
Table 3-11: Fractionation of methanol extract from Calothrix javanica biomass by RP-18 chromatography and antibacterial activity of fractions to S. aureus ..................... 82
Table 3-12: Step gradient used in HPLC analysis of CJFII by analytical HPLC ........... 83
Table 3-13: Separation of CJFII from Calothrix javanica biomass by semi-preparative reversed-phase HPLC and activity of the fractions to S. aureus ................... 84
Table 3-14: Step gradient used in HPLC analysis of seven fractions by analytical HPLC ................................................................................................................................ 84
Table 3-15: NMR data of CJFII-4..................................................................................... 86
Table 3-16: Sequence information deduced from the NOE’s found in the 2D NOESY spectrum of CJFII-4 ............................................................................................................ 88
Table 3-17: Calculation of the molecular mass from the structure of the residues deduced from the NMR data............................................................................................. 88

Table 3-18: Comparison of the sequence from the high-resolution ESI-MS data with that from the NMR data. ................................................................................................... 89
Table 3-19: Fractionation of methanol extract from Scytonema ocellatum biomass by RP-18 chromatography and antibacterial activity of fractions to S. aureus ................ 90
Table 3-20: Separation of SOFII from Scytonema ocellatum biomass by silical gel chromatography and antibacterial activity of the fraction to S. aureus ............................ 91
Table 3-21: Separation of SOFII-5 and SOFII-6 from Scytonema ocellatum biomass by semi-preparative reversed-phase HPLC and antibacterial activity of the fractions to S. aureus................................................................................................................................ 92
Table 3-22: Step gradient used in HPLC analysis of FSO3 by analytical HPLC........... 93
Table 3-23: Antibacterial activity of extracts from Anabaena sp. cultivated in large scale................................................................................................................................... 94
Table 3-24: Separation of EtOAc extract from Anabaena sp.culture medium by semi-preparative reversed-phase HPLC and antibacterial activity of the fractions to E. coli..................................................................................................................................... 95
Table 3-25: NMR data of AF6 (Fluourensadiol) ........................................................... 97
Table 3-26: Fractionation of methanol extract from Nostoc sp. biomass by silical gel chromatography and antibacterial activity of fractions to S. aureus ................................ 98
Table 3-27: Fractionation of NFIV from Nostoc sp. biomass by RP C18 chromatography and antibacterial activity of fractions to S. aureus ................................ 99
Table 3-28: Fractionation of NFIV-1 from Nostoc sp. biomass by semi-preparative RP HPLC and antibacterial activity of fractions to S. aureus .............................................. 100
Table 3-29: Fractionation of methanol extract from Lyngbya majuscula biomass by silica gel chromatography and cytotoxic activity of fractions against cell line 5637..... 101
Table 3-30: Separation of fraction F8 from Lyngbya majuscula biomass by silica gel chromatography and cytotoxic activity of fractions to 5637 cell line ............................ 102
Table 3-31: Separation of fraction F8-3 from Lyngbya majuscula biomass by preparative TLC.............................................................................................................. 103
Table 3-32: Comparison of 1H NMR data of compound 9 with reported data *.......... 104
Table 3-33: Fractionation of methanol extract from Lyngbya majuscula biomass by silica gel chromatography and cytotoxic activity of fractions against cell line 5637..... 106
Table 3-34 Separation of fraction F10 from Lyngbya majuscula biomass by semi-preparative RP HPLC and cytotoxic activity of fractions against cell line 5637 ........... 108
Table 3-35: Comparison of 13C NMR and 1H NMR data of compound 10 with literature values of *Debromoaplysiatoxin..................................................................... 109
Table 3-36: Comparison of 1H NMR data of compound 11 with reported data *........ 111
Table 3-37: Fatty acids analyzed as methyl ester in n-hexane extract.......................... 112

List of Schemes Scheme 2-1: Scheme of extraction................................................................................. 40
Scheme 2- 2: Extraction, fractionation, and isolation of the secondary metabolites of Westiellopsis sp.VN.......................................................................................................... 45
Scheme 2-3: Extraction, fractionation, and isolation of the secondary metabolites of Calothrix javanica ............................................................................................................ 46
Scheme 2-4: Extraction, fractionation, and isolation of the secondary metabolites of Scytonema ocellatum ........................................................................................................ 48
Scheme 2-5: Extraction, fractionation, and isolation of the secondary metabolites of Anabaena sp...................................................................................................................... 49
Scheme 2-6: Extraction, fractionation, and isolation of the secondary metabolites of Nostoc sp........................................................................................................................... 51
Scheme 2-7: Extraction, fractionation, and isolation of the secondary metabolites of Lyngbya majuscula (method 1)......................................................................................... 53
Scheme 2-8: Extraction, fractionation, and isolation of the secondary metabolites of Lyngbya majuscula (method2).......................................................................................... 55

Abbreviations
1D one-dimensional
2D two-dimensional
AS anisaldehyd- sulphuric acid
br broad
C18 octadecyl
CC column chromatography
COSY correlation spectroscopy
d doublet
DAD diot array detector
DCM dichloromethane
dd double of double
ddd doublet of doublet of doublets
DEPT distortionless excitation by polarization transfer
dq doublet of quartets
dt doublet of triplets
ESI electrospray ionization
EtOAc ethyl acetate
EtOH ethanol
GC-MS gas chromatography-mass spectrometry
Gln glutamine
HMBC heteronuclear multiple bond correlation
HMQC heteronuclear multiple quantum coherence
HPLC high performance liquid chromatography
HR-ESI-MS high resolution-ESI-MS
Hz hertz
IC50 50% inhibitory concentration
Ile isoleucine
INT iodonitrotetrazolium chloride
IR infrared
IZ inhibition zone
Leu leucine

m multiplet
m/z mass/charge
M+ molecular ion
Me methyl
MeCN acetonitrile
MeOH methanol
MHz megahertz
MS mass spectrometry
NMR nuclear magnetic resonance
NOE nuclear Overhauser effect
NOESY nuclear Overhauser effect spectroscopy
ppm part per million
Pro proline
q quartet
qd quartet of doublets
Rf retention factor
ROESY rotating frame nuclear Overhauser effect spectroscopy
rRNA ribosomal ribonucleic acid
s singlet
sp. species (singular)
SS solvent system
t triplet
TFA trifluroacetic acid
Thr threonine
TLC thin layer chromatography
TOCSY total correlation spectroscopy
TOF time of flight
tR retention time
Tyr tyrocine
UV ultraviolet
vis visible
δ chemical shift (in ppm)
PTLC preparative- TLC

Chapter I Introduction
1
1 Introduction
1.1 Cyanobacteria
1.1.1 Cyanobacterial physiology and morphology
Cyanobacteria, also known as blue-green algae, blue-green bacteria,
cyanoprokaryots, and cyanophytes, are oxygenic photosynthetic prokaryotes that
possess features familiar to both bacteria (prokaryota) and algae (eukaryota). Their
cell structure and composition are similar to those of prokaryotic cell in that they lack
the cell nucleus and distinctive organelles of eukaryotic cell, and their special
structure and chemical composition of the cell wall are basically the same as those of
gram-negative bacteria (Stanier & Cohen-Bazire, 1977; Van den Hoek et al., 1995;
Sakamoto et al., 1997; Castenholz, 2001; Kalaitzis et al., 2009). However, in contrast
to typical prokaryotes, they contain chlorophyll a and several accessory pigments
providing them with oxygenic photosynthetic ability like other algae, and their blue-
green color. They have two photosystems (PSII and PSI) and use water as an electron
donor during photosynthesis, leading to the production of oxygen. Several
cyanobacteria can also perform anoxygenic photosynthesis using only photosystem I
if electron donors such as hydrogen sulphide are present (Madigan et al., 2003). All
the known cyanobacteria are photoautotrophic, using primarily CO2 as carbon source
(Castenholz, 2001).
Cyanobacteria show considerable morphological diversity (Whitton &
Potts, 2000). They may either be unicellular, be aggregated into flat, spherical, regular
or irregular colonies, or form single filaments without branches or filaments with false
or true branching. Some cyanobacteria have the ability to produce two types of
specialized cells: (1) heterocysts, which provide the site for nitrogen fixation and
thereby counteract nitrogen demand under conditions of nitrogen deficiency, and (2)
akinetes, which are resting cells that allow the species to survive unfavourable growth
conditions. Many species of cyanobacteria possess gas vesicles, enabling them to
regulate their buoyancy and to maintain a certain vertical position in the water column
in response to physical and chemical factors (Reynolds, 1987; Walsby, 1994).
Asexual reproduction of cyanobacteria occurs by the formation of hormogonia
or endospores (exospores are modified endospores) or by fragmentation of colonies
(Lee, 1999)

Chapter I Introduction
2
1.1.2 Ecology of cyanobacteria
Cyanobacteria have a long evolutionary history and documented fossil records
date back to about 3500 million years ago (Schopf, 2000). However, the earliest
DNA-biomarker evidence suggests that cyanobacteria appeared about 2600 million
years ago (Hedges et al., 2001). It is widely accepted that ancient cyanobacteria
evolved oxygenic photosynthesis and played a major role in the change of the
oxygenless atmosphere to an oxygenic one (Schopf, 2000). Additionally,
cyanobacteria are believed to have had a considerable effect on the formation of
oxygen rich gas composition of Earth`s atmosphere (Dismukes, 2001; Paul, 2008),
and today the production of oxygen by cyanobacterial photosynthesis continues to
contribute to maintaining the balance of our atmosphere (Sielaft et al., 2006). Their
long evolutionary history is considered as a reason for the successful survival of
cyanobacteria in many habitats and their wide ecological tolerance (Whitton & Potts,
2000). In addition, cyanobacteria have developed a wide ecological tolerance to
temperature, light, salinity, moisture, alkalinity, and possess many characteristics and
adaptations that explain their world wide distribution and success. The distribution of
cyanobacteria is expanded widely on the earth in diverse ecosystems of marine,
freshwater, and terrestrial environments. They are most abundant in aquatic habitats
as part of the plankton, some can be found tightly or loosely attached to surfaces of
plants, rocks and sediments, and some can be found in hot and acidic springs, in salt
lakes, in deserts, ice shelves, and the arctic (Mur et al., 1999; Rastogi & Sinha, 2009).
Cyanobacteria are also important in many terrestrial environments and they can live in
soils or rocks and form symbiotic associations with plants, fungi and animals
(Whitton & Potts, 2000; Oren, 2000; Baracaldo et al., 2005; Thajuddin &
Subramanian, 2005).
The immense diversity within this group of microorganisms, apart from the
variability of morphology and range of habitats, is also reflected in the extent of their
synthesis of natural products. Cyanobacteria have evolved to produce a diverse array
of secondary metabolites that have aided species survival in these varied and highly
competitive ecological niches (Kalaitzis et al., 2009). Cyanobacteria are commonly
associated with the toxic blooms encountered in many eutrophic fresh and brackish
waters and are widely known for their potential to produce a range of neurotoxic,
hepatotoxic, and tumour promoting-secondary metablites (Codd et al., 1999; Sivonen
& Börner, 2008).
Cyanobacteria are unique phyla that grow in competitive niches and, as a
result, are promising sources of bioactive compounds (Clardy & Walsh, 2004; Lin et
al., 2008).

Chapter I Introduction
3
1.1.3 Classification of cyanobacteria
Taxonomic classification is a method for registration of the biodiversity and
the arrangement of individual into taxonomic groups. Therefore it should reflect
evolution, ecology, and phenotypic variations (Hoffmann et al., 2005). Taxonomic
classification of cyanobacteria is quite complex. There are presently two main
classification systems available (1) the botanical classification system (Komárek &
Anagnostidis, 1989; 1999; 2005 and Anagnostidis & Komárek, 1990) and (2) the
bacteriological classification system (Castenholz, 2001).
Cyanobacteria were traditionally classified on the basis of their morphology
only, according to the International Code of Botanical Nomenclature, ICBN (Greuter
et al., 2000). The taxonomy based on morphological characteristics alone does not
necessarily result in a phylogenetically reliable taxonomy despite the fact that the
cyanobacterial morphology is complex compared to most other prokaryotic microbes
(Giovanni et al., 1988; Wilmotte, 1994). Moreover, the problem of using only
morphological criteria is that some characters may vary considerable in response to
different environmental conditions making species delimitation difficult (Wilmotte &
Golubic 1991; Barker et al., 1999; Otsuka, 1999).
Cyanobacteria are also classified according to the International Code of
Nomenclature of Prokaryots, ICNP (Oren & Tindall, 2005). The bacteriological
classification is today widely based on phenotypic, chemotypic and genotypic
characteristics, the so called polyphasic approach, of pure culture of cyanobacteria. It
is a challenge to combine the traditional morphological classification and the
classification based on molecular methods, however, effort is made to unify these two
systems (Hoffmann, 2005; Oren & Tindall, 2005).
In the current botanical classification system, Komárek & Anagnostidis
(1989; 1999; and 2005) and Anagnostidis & Komárek, 1990) revised the taxonomy of
cyanobacteria and also included phenotypic and genotypic features. The botanical
approach distinguishes four orders of cyanobacteria (Komárek & Anagnostidis, 1989;
1999; 2005 and Anagnostidis & Komárek, 1990). The bacteriological taxonomic
system created for cyanobacteria is divided in five subsections (Rippka et al., 1979;
Castenholz, 2001) and they are to a large extent in agreement with the orders in the
botanical system (see table 1-1).
Ideally, taxonomy reflects evolutionary relationships of the classified
organisms, and the taxa are monophyletic groups of organisms (e.g. Wilmotte &

Chapter I Introduction
4
Golubic, 1991; Wilmotte, 1994). DNA sequences make it possible to infer
phylogenies of organisms (e.g. Moritz & Hillis, 1996) and DNA is not affected by
environmental factors in the same manner as many morphological traits are. The 16S
rRNA gene is universally present in bacteria and cyanobacteria and has a conserved
function. Woese and coworkers (Woese et al., 1976; Woese, 1987) established the
modern bacterial phylogenetic classification mainly based on the 16S rRNA gene
sequence. The widely used 16S rRNA region has been useful in several phylogenetic
analyses of cyanobacteria (e.g. Wilmotte& Golubic, 1991; Ben-Porath & Zehr, 1994;
Nelissen et al., 1996; Fergusson & Saint, 2000; Wilmotte & Herdman, 2001). Some
of the molecular methods have been also used for taxonomic studies of cyanobacteria
including DNA-DNA hybridization (Stam, 1980; Stam & Stulp, 1988; Wilmotte et
al., 1997), fingerprinting based on PCR with primers from short and long tandemly
repeated repetitive sequences (Rasmussen & Svenning, 1998), restriction fragment
length polymorphism (RFLP) (Mazel et al., 1990; Asayama et al., 1996; Lehtimäki et
al., 2000), DNA amplification methods (AFLP, ARDRA, REP-PCR, RAPD) (Neilan,
1995; Satish et al., 2001; Lyra et al., 2001), sequencing of marker genes, e.g. rpoC1
(Fergusson & Staint, 2000), nifH (Ben-Porath & Zehr, 1994), cpcB and cpcA (Manen
& Falquet, 2002; Ballot et al., 2004; Teneva et al., 2005), ITS region sequencing (the
internal transcribed spacer between the 16S rDNA and 23S rDNA) (Gugger et al.,
2002; Orcutt et al., 2002), PC-IGS region sequencing (phycocyanin operon intergenic
spacer) (Neilan et al., 1995; Bolch et al., 1996; Laamanen et al., 2001; Dyble et al.,
2002; Rohrlack et al., 2008).
Table 1-1: The orders of cyanobacteria according to the botanical classification and their correspondence to the subsections of the bacteriological code
Botanical
classification
Bacteriological
classification5
Main morphological features
and occurrence in the
environment
Example
Order
Chroococcales1
Subsection I Unicellular cyanobacteria that
reproduce by binary cell division
in one, two or three plane; or
budding, either single cells or in
colonies held together by
Synechocystis
Microcystis

Chapter I Introduction
5
mucilage or laminated sheaths.
Many species are planktonic and
contain gas vesicles. They are
widespread in freshwater,
brackish water and marine
environment.
Subsection II Unicellular or non-filamentous
aggregates of cells held together
by outer wall or gel-like matrix.
Some species can sometimes or
always reproduce by small
spherical cells (baeocytes) which
are produced by multiple
divisions of the mother cells.
They generally grow in aquatic
environments attached to
substrata.
Pleurocapsa
Order
Oscillatoriales2 Subsection III Organisms form trichomes of
vegetative cells, mostly
uniseriate, without differentiated
cells such as heterocytes and
akinetes (resting cells, spores).
The trichomes show not true
branches but in some genera false
ramifications may occur. The
trichomes usually have a sheath
and many species have gas
vesicles. Cell division occurs
always in one plane perpendicular
to the longitudinal axis of
trichome.
The group is ecologically diverse
and they live in plankton, benthic
and periphytic in freshwater and
in marine environments.
Oscillatoria
Spirulina
Lyngbya
Planktothrix
Limnothrix

Chapter I Introduction
6
Order
Nostocales3 Subsection IV Binary division in one plane
giving rise to 1-seriate trichomes,
though sometimes with "false"
branches; one or more cells per
trichome differentiate into
heterocysts, at least when
concentration of nitrogen is low;
some also produce akinetes; some
may form hormogonia (formation
of motile trichomes that give rise
to young filaments).They occur in
plankton, benthic and periphytic
in freshwater, marine, and
terrestrial environments.
Anabaena
Nodularia
Nostoc
Scytonema
Calothrix
Order
Stigonematales4 Subsection V Binary division periodically or
commonly in more than one
plane, giving rise to multiseriate
trichomes, or trichomes with true
branches or both; apparently
always possess ability to form
heterocysts; some also form
akinetes; in some genera, there is
a differentiation into main
filament and branches. They
occur in terrestrial and aquatic
environments but usually not in
the plankton.
Fischerella
Hapalosiphon
Westiellopsis
1. Komárek & Anagnostidis, 1999; 2. Komárek & Anagnostidis, 2005; 3. Komárek & Anagnostidis,
1989; 4. Anagnostidis & Komárek, 1990; 5. Rippka et al., 1979, Castenholz, 2001
1.2 Cyanobacteria–a new and rich source of novel bioactive
compounds with pharmaceutical potential
Natural products (secondary metabolites) are an important source of new
pharmaceuticals and pharmaceutical 'lead' compounds. Natural products serve not
only as drugs in their own right, but they may also serve as structural models for the

Chapter I Introduction
7
creation of synthetic analogues, and as models in structure-activity studies (e.g. Quinn
et al., 1993; Harvey, 2008; Gademann and Kobylinska, 2009). Of the 974 small
molecule new chemical entities introduced as drugs worldwide during 1981-2006,
63% were inspired by natural products (Newman & Cragg, 2007). These include
natural products (6%), natural product derivatives (28%), synthetic compounds with
natural product derived pharmacophores (5%) and synthetic compounds based on
knowledge gained from a natural product (natural product mimic, 24%). In certain
therapeutic areas the productivity was higher: 77.8% of anticancer drugs are either
natural products or derived from natural products. The overwhelming majority of
active compounds have been derived from streptomycetes, fungi, bacteria and plants.
A major problem in focusing on these sources in the search for novel, biologically
active molecules, is the rediscovery of previously known natural products. One way to
minimize this problem is to develop selective bioassays and investigate new
therapeutic areas. Another approach is to look at new and different sources of natural
products. Natural products have been isolated from a wide variety of taxa and tested
for various biological activities. Among these taxa, cyanobacteria represent such a
source. They have been identified as a new and rich source of bioactive compounds
(Abarzua et al., 1999; Burja et al., 2001; Shimizu, 2003; Bhadury et al., 2004;
Wiegand & Pflugmacher, 2005; Dahms et al., 2006; Tan, 2007; Jaiswal et al., 2008;
Smith et al., 2008; Sivonen & Börner, 2008; Gademan & Portmann, 2008).
Numerous bioactive compounds isolated from different cyanobacterial strains
exhibited novel and a diverse range of biological activities and chemical structures
including novel peptides (e.g. cyclic depsipeptides, cyclic peptides, lipopeptides),
fatty acids, polyketides, alkaloids, amides, terpenes, carbohydrates, and other organic
chemicals (Patterson et al., 1994; Namikoshi & Rinehart, 1996; Abarzua et al., 1999;
Kreitlow et al., 1999; Burja et al., 2001; Singh et al., 2005;Welker &VonDöhren,
2006; Sielaff et al., 2006; Spolaore et al., 2006; Ramaswamy et al., 2006; Wagoner et
al., 2007 ; Tan, 2007; Baumann, 2007; Blunt et al., 2008, 2010; Portmann et al.,
2008; Medina et al., 2008;Gademann & Portmann, 2008 ; Van Wagoner et al., 2007;
Tripathi et al., 2009; Tidgewel et al., 2009).
Many of these are regarded as good candidates for drug discovery, with
applications in agriculture (Biondi et al., 2004), industry (De Philippis et al., 1998;
Spolaore et al., 2006; Jaiswal et al., 2008; Rastogi &Sinha, 2009), and especially, in
pharmacy (Mundt et al., 2001; Singh et al., 2005; Sielaff et al., 2006; Dunlap et al.,

Chapter I Introduction
8
2007; Tan, 2007; Gademann & Portmann, 2008; Gerwick et al., 2008; Rastogi &
Sinha, 2009).
The cyanobacterial bioactive compounds provide novel and useful
pharmaceuticals that are difficult to produce synthetically because of their structural
complexity (Kaushik & Chauhan, 2009). The chemical diversity and novelty seen in
cyanobacteria are comparable to those of Actinomycetes, which have turned out many
important drugs. It is not unusual that a single species produces many different
chemotypes. Lyngbya majuscula is a good example. The diversity of structures found
in this ubiquitous filamentous cyanobacterium is just incredible. Compounds isolated
from this strain are lipopeptides (cyclic or linear), amino acids, lactones, fatty acids,
amides, alkaloids, pyrroles, depsipeptides and many others (Burja et al., 2001;
Shimizu, 2003; McPhai et al., 2007; Tripathi et al., 2009; Gutiérrez et al., 2008; Tan,
et al., 2008; Jones et al., 2009, 2010; Blunt et al., 2010 ). Most of them possess
characteristic biological activity. For example, curacin A isolated from a Cuasao
strain by Gerwick's group (Gerwick et al., 1994b) was recognized as a new
pharmacophore to perturb microtubule assembly and acts as potent antimitotic agent.
With a rather simple structure, it is an important lead compound for new types of
anticancer drugs.
The relative disregard in the past of cyanobacteria compared with other
microbial sources of natural products, and the microbial diversity of cyanobacteria, as
well as the huge chemical and biologically active diversity of their products
recommend them as an attractive source of novel drugs for use in diverse therapeutic
areas and should imply opportunity for a most significant progress in the generation
of novel bioactive substances. Furthermore, cyanobacteria also have the advantage
over many other organisms as they can be cultured, thus providing an alternative to
chemical synthesis for the production of their bioactive compounds.
Cyanobacterial metabolites show an interesting and exciting range of
biological activities ranging from antimicrobial, anticancer, antiviral,
immunosuppressant, insecticidal, anti-inflammatory to proteinase-inhibiting activities
which are striking targets of biomedical research (Borowitzka, 1995; Kulik, 1995;
Luesch et al., 2002; Soltani et al., 2005; Tan, 2006; Dunlap et al., 2007; Wase &
Wright, 2008; Gerwick et al., 2008; Abed et al., 2009, Gademann & Kobylinska,
2009; Villa et al., 2010).

Chapter I Introduction
9
1.2.1 Antimicrobials
Cyanobacteria have been shown to be a source of antibiotic compounds
(e.g.Harvey, 2000; Jaki et al., 1999b; Burja et al., 2001; Volk &Furkert, 2006;
Chlipala et al., 2009).
Most of the antibiotic metabolites isolated until now were accumulated in
the cyanobacterial biomass, but cyanobacteria are also known to excrete various
antibiotic compounds into their environments (Moore et al., 1984a; De Caire et al.,
1993; Jaki et al., 1999a and 2000a; Volk 2006; Jaiswal et al., 2008). In spite of the
studies carried out so far, many cyanobacterial compounds are still largely unexplored
and the chemicals involved are mostly unidentified, thus giving a rich opportunity for
discovery of new bioactive compounds. The antibiotic activities include antibacterial,
antifungal, and algicidal activity etc.
Secondary metabolites with antibacterial activity are widely produced by
cyanobacteria. These compounds are effective against Gram-positive and/or Gram –
negative bacteria, however, it has been found that the antibacterial activity of
cyanobacteria is mainly directed against Gram-positive bacteria since most Gram-
negative bacteria are resistant to toxic agents in the environment due to the barrier of
lipopolysaccharides on their outer membrane (Dixon et al., 2004). Both toxic and
nontoxic strains of cyanobacteria are producers of antibacterial compounds that are
distinct from cyanotoxins (Østensvik et al., 1998). Antibacterial effects of extracts
from Fischerella sp. (Asthana et al., 2006), Spirulina platensis (Kaushik &Chauhan,
2008; Abedin &Taha, 2008), Anabaena variabilis (Kaushik et al., 2009), Nostoc sp.
CCC537 (Asthana et al., 2009), Oscillatoria (Shanab, 2007), Anabaena and Nostoc
(Svircev et al., 2008), Synechocystis and Synechococcus (Martins et al., 2008) and
other species belonging to the orders of Chroococales, Pleurocapsales, Oscilatoriales,
Nostocales, Stigonematales (Soltani et al., 2005; Taton et al., 2006; Chlipala et al.,
2009; Patil et al., 2009) have been reported. Up to now, there have been published
several reports of antibacterial compounds isolated from cyanobacteria, such
examples as ambiguine isonitriles, aoscomin, comnostins A-E, norharmane,
lyngbyazothrins, carbamidocyclophanes (Smitka et al., 1992; Jaki at el., 1999a and
2000a,b; Volk & Furkert, 2006; Bui et al., 2007; Raveh & Carmeli, 2007; Zainuddin
et al., 2009; Mo et al., 2009).
Several extracts of cyanobacteria belong to Stigonematales, Nostocales and
Oscillatoriales have shown antifungal activity in in-vitro test systems (Moore et al.,

Chapter I Introduction
10
1987; Parket et al., 1992; Smitka et al., 1992; Stratmann et al., 1994; Soltani et al.,
2005; Pawar &Puranik, 2007; Abedin & Taha, 2008; Svircev et al., 2008). Antifungal
compounds isolated from these extracts include hapalindoles, tolytoxin, scytophycins,
toyocamycin, tjipanazoles, hassallidin A, nostocyclamide and nostodione (Abed et al.,
2009).
Antibacterial and antifungal substances identified also include fatty acids
(Gerwick et al.,1987; Mundt et al., 2003; Asthana et al., 2006), phenolics (DeCano et
al.,1990), bromophenols (Pedersén & DaSilva, 1973), terpenoids (Jaki et al.,
2000a,b), N-glycosides (Bonjouklian et al., 1991), cyclic depsipeptides (Carter et
al.,1984), lipopeptides (MacMillan et al., 2002; Neuhof et al., 2005), cyclic peptides
(Pergament &Carmeli, 1994), cylic undecapeptides (Zainuddin et al., 2009) and
isonitrile-containing indole alkaloids (Moore et al.,1987; Smitka et al.,1992; Raveh
& Carmeli, 2007, Mo et al., 2009).
Relatively little has been known on the antimicrobial activity of
cyanobacterial compounds from Chroococcales group (e.g., Synechoccystis and
Synechoccocus), however, Martins et al., (2008) emphasise the potential of these
genera as a source of antibiotic compounds that produce substances with inhibitory
effects on prokaryotic cells and with apoptotic activity in eukaryotic cell lines, which
highlights the importance of these organisms as potential pharmacological agents.
Unfortunately, almost all of the studies have used only in vitro assays, it is
likely that most of compounds responsible for antibiotic activity will have little or no
application in medicine as they are either too toxic or are inactive in vivo ( Reichelt &
Borowitzka, 1984; Borowitzka, 1995). They may, however serve as useful leads to
create new synthetic antibiotics or may find application in agriculture. For example,
cryptophycin 1 was first isolated from Nostoc sp. ATCC 53789 by the Merck's group
as a fungicide (Schwartz et al., 1990). However, it was found to be too toxic to use as
an antifungal agent and no further studies were carried out with this compound.
Subsequently, Moore and co-workers isolated this same compound from Nostoc sp.
GSV 224, which exhibited powerful cytotoxicity against human tumor cell lines and
good activity against a broad spectrum of drug-sensitive and drug-resistant murine
and human solid tumors. Nevertheless, cryptophycin 1 again appeared to be too toxic
to become a clinical candidate. This led to a detailed structure-function study by
Moore in collaboration with Carmichael, and resulted in creation of cryptophycin 8
(see fig.1-1) a semisynthetic analogous which proved to have a greater therapeutic

Chapter I Introduction
11
efficiency and lower toxicity than cryptophycin 1 in vivo (Moore et al., 1996; Liang et
al., 2005).
Figure 1-1: Crytophycin 1 and crytophycin 8
It has also been shown that cyanobacteria produce a broad spectrum of
antialgal compounds, which may be used to control cyanobacteria and algal blooms.
Cyanobacteria probably use these compounds in order to out-compete other micro-
organisms, to gain dominance over other organisms, or influence the type of
conspecifics and successors (Gross, 2003; Dahms et al., 2006). A growing number of
studies have identified cyanobacterial metabolites that act as algaecides (Mason et al.,
1982; Vepritskii et al., 1991; Gromov et al., 1991; Schlegel et al., 1999; Smith &
Doan, 1999; Volk & Furkert, 2006; Shanab, 2007; Berry et al., 2008). Mason et al.,
1982 reported the identification and characterization of cyanobacterin (chlorinated γ-
lactone) from the filamentous freshwater cyanobacterium Scytonema hofmanni that
specifically inhibited a range of algae, including other cyanobacteria and green algae,
at micromolar concentrations, but had little effect on non-photosynthetic microbes. It
was later found that cyanobacterin specifically inhibits photosystem II (Gleason
&Case, 1986). Compounds such as cyanobacterins LU-1 and LU-2 (Gromov et al.,
1991; Vepritskii et al.,1991; Ishibashi et al., 2005) were reported from Nostoc linckia
that are structurally different from cyanobacterin but specifically inhibit transport of
electrons in photosystem II. It was found that LU-1 inhibited cyanobacteria as well as
algae but not photosynthetic microbes, whereas LU-2 inhibited cyanobacteria only.
Flores & Wolk, 1986 and Schlegal et al., 1999 independently screened 65 and
approximately 200 isolated of cyanobacteria, respectively, for algaecidal activity.
Interestingly, it was found in these studies that antialgal activity was largely restricted
to several genera, namely Fischerella, Nostoc, Anabaena, Calothrix and Scytonema.
Fischerella produces the fischerellins and hapalindoles, which both inhibit
photosynthesis and RNA polymerization (Srivastava et al., 1999; Gantar et al., 2008).

Chapter I Introduction
12
The bioactive compounds of Oscillatoria species also showed antibiotic activity
against green algae and cyanobacteria, including a toxic Microcystis aeruginosa
species (Smith & Doan, 1999; Shanab, 2007). More recently, Berry et al., 2008
screened 76 isolates of cyanobacteria from the Everglades for antialgal activity
against two sympatric representatives of green algae (Selanstrum 34-4 and
Chlamydomonas Ev-29) and cyanobacteria (Anabaena 66-2 and Synechococcus 40-
4). Of these, 40 isolates (53% of those tested) inhibited one or more of the
representative strains. It has been described that the pentacyclic calothrixins, isolated
from Calothrix (Rickards et al. 1999), inhibit RNA polymerase and DNA synthesis,
and hence act as allelopathic compounds (Doan et al., 2000). In addition to
cyanobacterins LU-1 and LU-2, the genus Nostoc produces several other metabolites
that have been associated with algaecidal activity e.g., nostocyclamide, nostocine A as
well as nostocarboline (Todorova et al., 1995; Hirata et al., 2003; Blom et al., 2006).
It has been reported that the microcystins (e.g. microcystin-LR) isolated from M.
aeruginosa inhibit the growth of cyanobacteria, including Nostoc, Synechococcus and
Anabaena species (Singh et al., 2001). Recently, an interesting compound,
norharmane (see fig.1-2) from Nodularia harveyana exhibited anticyanobacterial
activity against both filamentous and unicellular cyanobacteria and may be used for
the control of toxic algal blooms (Volk, 2006).
Figure 1-2: Norharmane from cyanobacteria
1.2.2 Cytotoxic and antitumoural activities
Blue-green algae have been found to be excellent sources of new anticancer
agents (Moore et al., 1988; Patterson et al., 1991; Gerwick et al., 1994a; Burja et al.,
2001; Simmons et al., 2005; Tan, 2007; Sivonen & Börner, 2008; Gerwick et al.,
2008). Freshwater and marine cyanobacteria are well recognized for producing

Chapter I Introduction
13
numerous and structurally diverse bioactive and cytotoxic secondary metabolites
suited to drug discovery (Dunlap et al., 2007).
Researchers found that a relatively high percentage of extracts from
cultured cyanobacteria showed cytotoxicity, and was active in vivo. 6% of extracts of
over 1000 cultured cyanobacterial strains showed cytotoxicity against the KB cell line
(a human epidermoid carcinoma of the nasopharynx) with MICs< 30µg/ml) (Moore et
al.,1988). According to Patterson et al., 1991, in their large-scale screening program
initiated to evaluate laboratory-cultured blue-green algae (cyanobacteria) as a source
of novel antineoplastic agents, approximately 1000 cyanophyte strains from diverse
habitats were cultured to provide extracts for testing. This screening program showed
approximately 7% of extracts tested inhibited proliferation of the KB cell line, the
families Scytonemataceae and Stigonemataceae as prolific producers of novel
cytotoxic compounds, and rates of rediscovery of known compounds were relatively
low.
Research of Gerwick et al., 1994b at Oregon State University had focused
on marine cyanobacteria and primarily screening of compounds for anticancer
activity. This work had clearly shown that marine cyanobacteria are an exciting
source of novel bioactive compounds, with a high number of purified metabolites
demonstrating activity. Interestingly, approximately 40% of the marine cyanobacterial
compounds possess anticancer/antitumor activity, making them invaluable as
potential therapeutic leads (Jaspars & Lawton, 1998; Tan, 2007). Marine
cyanobacteria are also an exceptionally rich source of novel peptides and integrated
peptide-polyketide type natural products. Many of these natural products are potently
cytotoxic to mammalian cells, and this has furthered their exploration as a source of
new anticancer lead compounds (Gerwick et al., 2008).
To date, many researches have focused on screening for anticancer
compounds (e.g. Martin et al., 2008; Sivonen et al., 2008) and a variety of cytotoxic
compounds have been isolated from cyanobacteria, many of them possess
unprecedented structures and therefore have the potential for development of entirely
new classes of drug agents.
Perhaps the cryptophycins are among the earliest and most prominent
candidates for new anticancer drugs. When the cryptophycins, a group of >25
cyanobacterial metabolites with strong tubulin-destabilizing activities (Smith et al.,
1994; Panda et al., 1997; Corbett et al., 1997; Eggen & Georg, 2002), were

Chapter I Introduction
14
discovered, hopes were great that one of these natural products could be developed
into a useful anticancer drug. In fact, the prototype cryptophycin 1, mentioned in
1.2.1, of this class of natural anticancer drugs from the cyanobacterium Nostoc sp.
ATCC 53789, is one of the most potent tubulin-destabilizing agents ever found
(Smith et al., 1994). In addition, the cryptophycins, like the epothilones, were not
substrates of P-glycoprotein, an efflux pump that makes multidrug-resistant cancer
cell lines immune against a multitude of anticancer drugs (Smith et al., 1994; Fojo et
al., 2005; Breier et al., 2005). Consequently, cryptophycin 52 (see fig. 1-3), a
synthetic analogue, was developed and reached phase II of clinical trials (Sessa et al.,
2002; Edelman et al., 2003; D'Agostino, 2006). The synthetic analogue 52 was chosen
because no large-scale biotechnological production method existed for the
cryptophycins. Eventually, the high production costs and toxic side effects of
cryptophycin 52 stopped its development and that of any other analogues of the
cryptophycin family. Nobody wanted to restart all of the trials with a different
analogue, although preclinical studies showed that other analogues would have been a
better choice (Liang et al., 2005). Nonetheless, studies to find new cryptophycin
analogues or to develop semisynthetic/biotechnological methods for the generation of
promising cryptophycin analogues continued (Liang et al., 2005; Beck et al., 2005).
To date, the research group of David H. Sherman of the University of Michigan in
collaboration with Richard E. Moore of the University of Hawaii (Magarvey et al.,
2006) has studied in a very comprehensive way the biosynthesis of the cryptophycins
and cryptophycin 52 is now biotechnologically producible, which may make this drug
more easily accessible and less costly to produce if further pursued (Rohr, 2006).
Figure 1-3: Cryptophycin 1 and 52, potent antitumor agents from cyanobacteria

Chapter I Introduction
15
In addition, an increasing number of marine cyanobacteria are found to target
tubulin or actin filaments in eukaryotic cells, making them an attractive source of
natural products as anticancer agents (Jordan &Wilson, 1998). Prominent compounds
isolated from marine cyanobacteria such as the anti-microtubuli agents, curacin A (1),
dolastatin 10(2), and dolastatin 15 have been in preclinical and /or clinical trials as
potential anticancer drugs (Gerwick et al., 2001; Newman & Cragg, 2004; Simmons
et al., 2005; Tan, 2007; Butler, 2008). Many of marine cyanobacterial molecules with
potent biological activities are also lead compounds for the development of synthetic
analogs having increased potency and decreased toxicity. As shown in figure 1-4.
Curacin A (1), dolastatin 10(2), and dolastatin 15 (3) served as lead structures for the
development of synthetic analogues. e.g. compound 4, TZT-1027 (5), ILX-651 (6),
and LU-103793 (7), usually with improved pharmacokinetic properties (Wipf et al.,
2004; Mita et al., 2006; Wantanabe et al., 2006; Tan, 2007; Butler, 2008).
Figure 1-4: Prominent anticancer marine cyanobacterial secondary metabolites and synthetic
analogues (Tan 2007)
Curacin A (1) a metabolite isolated from a Curaçao strain of the marine
cyanobacterium Lyngbya majuscula (Gerwick et al.,1994b) exhibits potent anti-
proliferative and cytotoxic activity against colon, renal, and breast cancer derived cell

Chapter I Introduction
16
lines but is effectively insoluble in any formulation and thus has not been reported to
produce activity in in vivo animal models. However, as a lead molecule, it has
inspired the synthetic production of numerous analogs, some of which show potent
cytotoxic effects but with increased stability and water solubility (Wift et al., 2004),
e.g. compound (4), a synthetic analog of curacinA, is undergoing evaluation ( Tan,
2007; Gerwick et al., 2008; Jones et al., 2009).
Dolastatin 10 (2) was first isolated in 1987 from the Indian Ocean sea hare,
Dolabella auricularia (Pettit et al., 1987), and then isolated from the marine
cyanobacterium Symploca (Luesch et al., 2001). It possesses potent antiproliferative
activity in vitro against a variety of human leukemias, lymphomas, and solid tumor
cell lines (Pezer et al., 2005; Simmons et al., 2005). Indeed, while dolastatin 10 is no
longer a clinical trial agent derivatives such as TZT-1027 (soblidotin) (5) are still
being evaluated (http://www.clinicaltrials.gov/ct2/results?term=soblidotin; Patel et
al., 2006; Gerwick et al., 2008; Butler, 2008).
Dolastatin 15 (3) was initially isolated from extracts of the Indian Ocean
sea hare D. auricularia and numerous dolastatin 15–related peptides have been also
isolated from diverse marine cyanobacteria (Beckwith et al., 1993; Gerwick et al.,
2001), Dolastatin 15 inhibits proliferation of human malignant cell lines in vitro and is
active in a broad range of animal tumor models (Hamel et al., 2002; Ray et al., 2007).
Obstacles to further clinical evaluation of dolastatin 15 include the complexity and
low yield of its chemical synthesis and its poor water solubility. However, these
impediments have prompted the development of various synthetic analogue
compounds with enhanced chemical properties, including ILX-651 (6) and LU103793
(7) (Simmons et al., 2005; Ray et al., 2007). ILX-651(tasidotin or synthadotin) (6) is
an orally active third generation synthetic dolastatin 15 (3) analogue, ILX-651 is
currently undergoing Phase II trials after its successful run in Phase I trials (Simmons
et al., 2005 ; Mita et al., 2006; Ray et al., 2007; Dunlap et al., 2007; Butler, 2008;
http://www.clinicaltrials.gov/ct2/results?term=synthadotin)
Belamide A, a highly methylated linear tetrapeptide related to the dolastatin
family of potent anticancer agents, was purified from a Panamanian marine
cyanobacterium Symploca sp. This compound contains two characteric residues, the
N-terminal N, N-dimethylvaline and C-terminal benzyl (methoxy) pyrrolinone
moieties (Simmons et al., 2006). Belamide A demonstrated cytotoxicities against the
MCF7 breast cancer-and the HCT-116 colon cancer cell lines, and displayed classic

Chapter I Introduction
17
microtubule-depolymerizing effects in A-10 cells, including concentration-dependent
interphase microtubule loss, micronucleation and abnormal mitotic spindle formation.
Therefore, belamide A may provide a valuable starting point for structure- activity
relationship (SAR) studies (Baker et al., 2007).
Other noteworthy marine cyanobacterial molecules reported in the literature
having significant cytotoxic activity include borophycin and apratoxin A. Borophycin
(see fig.1-5) is a complex boron containing polyketide isolated from marine strains of
Nostoc linckia and Nostoc spongiaeforme var. tenue (Banker and Carmeli, 1998).
Borophycin, which is related both to the boron-containing boromycins isolated from a
terrestrial strain of Streptomyces antibioticus and to the aplasmomycins isolated from
a marine strain of Streptomyces griseus (actinomycetes), exhibits promising antitumor
activity against standard cancer cell lines (MIC 0.066mg/mL, LoVo and 3.3mg/mL
KB), and human epidermoid carcinoma and human colorectal adenocarcinoma cell
lines (Davidson, 1995; Banker et al., 1998; Gademann &Portmann, 2008).
Figure 1-5: Borophycin from cyanobacteria
Apratoxin A first isolated from Lyngbya majuscula found at Apra Harbor,
Guam, is a potent cytotoxin with a unique carbon skeleton. It possesses an impressive
biological profile in in vitro cytotoxicity assays against various human tumor cell
lines with IC50 values ranging from 0.36 to 0.52 nM. Total synthesis of apratoxin A
has been accomplished leading the way to synthetic analogs for the purpose of new
therapeutics (Chen & Forsyth, 2004).
Recently, several of potent anticancer marine cyanobacterial metabolites
have been published including grassypeptolide, aerucyclamide A and B, hantupeptin
A, and Apratoxins D and E (Kwan et al., 2008; Portmann et al., 2008; Gutiérrez et al.,
2008; Matthew et al., 2008; Tripathi et al., 2009).

Chapter I Introduction
18
1.2.3 Antiviral activity
Cyanobacteria also appear to be a rich source of new antiviral compounds.
The initial screening program by Rinehart et al., 1981 indicated that a large
percentage of extracts of field- collected cyanophytes exhibited antiviral activity when
assayed against herpes simplex virus, type II (HSV-2). Then, screening programs of
the University of Hawaii and the U.S. National Cancer Institute have demonstrated
antiviral activity in approximately 10% of extracts tested (some 600 cyanophyte
strains) using live virus test systems for inhibition of HSV-2 and human
immunodeficiency virus, type 1 (HIV-1) whereas a smaller percentage (2.5%) of the
extracts were active against respiratory syncytial virus (Patterson et al., 1993). Lau et
al., 1993 have also screened extracts of over 900 strains of cultured cyanobacteria for
inhibition of the reverse transcriptases (RT) of avian myeloblastosis virus (AMV) and
human immunodeficiency virus, type 1 (HIV-1), and they found that over 2% of
extracts showed activity against AMV and HIV RTs.
Few of the antiviral compounds were isolated from cyanobacteria so far.
Bioassay-directed fractionation of the National Cancer Institute of cyanophytes grown
at the University of Hawaii led to the isolation of a family of anti–HIV sulfolipids
(Gustafson et al., 1989; Patterson et al., 1994). Other compounds were anti-HSV-2
indolocarbazoles from Nostoc sphaericum (Knübel et al., 1990). Ambiguol A from
Fischerella ambigua has been shown to inhibit HIV-1 reverse transcriptase and
cyclooxigenase (Falch et al., 1993).
A sulphated polysaccharide isolated from Spirulina platensis (Hayashi et al.,
1993 and 1996) named calcium spirulan, inhibits replication in enveloped viruses
such as HSV-1, HIV-1, human cytomegalovirus, etc. This polysaccharide is
composed of rhamnose, ribose, mannose, fructose, galactose, xylose, glucose,
glucuronic acid, sulphate and calcium, and the calcium appears to be essential for
maintaining the replication-inhibiting activity. Nostoflan isolated from Nostoc
flagelliforme (Kanekiyo et al., 2005) and ichthyopeptins A and B isolated from
Microcystis ichthyoblabe (Zainuddin et al., 2007) exhibited antiviral activity.
Two interesting antiviral compounds also have been isolated from
cyanobacteria: cyanovirin-N from Nostoc ellipsosporum (Boyd et al., 1997) and
scytovirin from Scytonema vatium (Bokesch et al., 2003). Cyanovirin-N, a novel 11
kDa protein, inactivates the human immunodeficiency virus (HIV) and has high
potency against most strains of influenza A and B viruses (O’Keefe et al., 2003;

Chapter I Introduction
19
Sivonen & Börner, 2008; Xiong et al., 2010). Cyanovirin –N is under development as
an antiviral agent, thanks to its effectiveness against HIV, its non-toxicity to human
cells, and its persistence (Bewley et al., 1998). Currently, cyanovirin-N is available as
a vaginal gel for local protection to HIV infection (http://www.aidsinfo.nih.gov). In
addition, a recent ex vivo test showed that the antiviral effect of cyanovirin-N is
stronger than that of PRO 2000 (Fischetti et al., 2009; Huskens et al., 2009), a
nonspecific polyanion microbicide. Scytovirin is a protein that acts similarly to
cyanovirin –N, but is less efficient in inactivation of viruses (Bokesch et al., 2003,
Xiong et al., 2006).
The potential of these drug candidates to achieve clinical success holds
great strategic promise for exploiting the structural complexity of cyanobacterial
metabolites across diverse therapeutic areas in future drug discovery (Sielaff et al.,
2006).
1.2.4 Toxins and other pharmacologically active compounds
Cyanobacteria are better known as producers of highly toxic compounds
(cyanotoxins) (Dow & Swoboda, 2000; Codd et al., 2005; Sivonen & Börner, 2008;
Jaiswal et al., 2008; Stewart et al., 2009). Cyanotoxins are bioactive secondary
metabolites produced by cyanobacteria and the majority are commonly grouped
according to their physiological effects either as cytotoxins (e.g. cryptophycins,
dolastatins, symplostatins), neurotoxins (e.g. anatoxins, saxitoxins), hepatotoxins (e.g.
microcystins, nodularins), or as irritants and gastrointestinal toxins (e.g. aplysiatoxins
and lyngbyatoxin). Up to now, some of cyanobacterial toxins are known as
allelochemicals with potential applications as algaecides, herbicides and insecticides
(Berry et al., 2008), and many of cytotoxins isolated from cyanobacteria have
potential as anticancer drugs (Gerwick et al., 1986 and 1989; Moore et al., 1988;
Borowitzka, 1995; Van Wagoner et al., 2007; Gademann & Portmann, 2008), some of
them are discussed in 1.2.2.
The cyanobacteria have also showed to be a rich source of highly effective
inhibitors of proteases (Itou et al., 1999; Borowitzka, 1999; Hee et al., 2008). Since
proteases are involved in a variety of biological processes, and many proteases are
validated drug targets (Turk, 2006), the discovery of new protease modulators is
important to the development of pharmacological tools as well as potential
therapeutics. For example, cancer cells are more sentitive to the proapoptotic effects

Chapter I Introduction
20
of proteasome inhibition than normal cells. Thus, proteasome inhibitors can be
potential anticancer agents. Protease inhibitors can be produced by both toxic
cyanobacterial strains (e.g., those that produce hepatotoxins or neurotoxins) and non-
toxic cyanobacterial strains of Microcystic, Anabaena, Planktothrix/Oscillatoria and
Nostoc (Smith et al., 2008). Several of protease inhibitors isolated from
cyanaobacteria have been published including aeruginopeptins, anabaenopeptilides,
cyanopeptins, micropeptins, nostopeptins, oscillapeptins, miroviridins, aeruginosins,
microcins, anabaenopeptins, oscillamides (Welker & van Döhren, 2006; Smith et al.,
2008), banyasin A (Plouno et al., 2005), largamides A-H (Plaza & Bewley, 2006),
lyngbyastatins 4-7 (Matthew et al., 2007; Taori et al., 2007), planktocyclin
(Baumann et al., 2007), kempopeptins A and B (Taori et al., 2008), nostodione A
(Hee et al., 2008) and others. In addition, some protease inhibitors may also find
application in medicine for treatment of stroke, coronary artery occlusions and
pulmonary emphysema. For example, inhibitors of the serine protease, thrombin,
could be used to control blood clot formation in these diseases. Thrombin acts by
cleaving a peptide fragment from fibrinogen which then leads to the formation of
fibrin, a major component of blood clots. Inhibition of this protease would thus also
inhibit clot formation. Similarly, angiotensin-converting enzyme inhibitors are being
developed as anti-hypertensive agents. Protease inhibitors are also used in the
treatment of HIV infections (Richman, 1996). These inhibitors include linear and
cyclic peptides as well as depsipeptides and have been isolated mainly from
Microcystis and Oscillatoria strains (Borowitzka, 1999).
Some of cyanobacterial metabolites have promising therapeutic applications
showing anti-inflammatory activities, as for example, malyngamide F acetate isolated
from Lyngbya majuscula exhibited strong concentration-dependent anti-inflammatory
activity in the nitric oxide (NO) assay with an IC50 of 7.1 µM and with no cytotoxicity
at the concentrations tested (Villa et al., 2010).
Haploindolone A and B, isolated from the cyanobacterium Fischerella
(ATCC 53558), have been patented as vassopressin antagonists with possible
application in the treatment of congestive heart failure, hypertension, oedema and
hyponatriaemia (Schwartz et al., 1989).
There are also several reports of cyanobacterial extracts acting on the
immune system. For example, extracts of Spirulina platensis have been shown to

Chapter I Introduction
21
enhance chicken macrophage function in vitro as well as cell-mediated immune
function in chickens and cats (Qureshi et al., 1995 and 1996; Qureshi & Ali, 1996).
Other interesting activities such as antimalarial (McPhail et al., 2007;
Gademann & Kobylinska, 2009), immunosuppressant (Koehn et al., 1992; Zang et
al., 1997), insecticidal activity (Beche et al., 2007), and antiplasmodial (Papendorf
et al., 1998; Barbaras et al., 2008) isolated from cyanobacteria have been also
reported.
Recently, it has also been found that the carbolinium alkaloid,
nostocarboline (see fig.1-6) isolated from Nostoc 78-12A (Becher et al., 2005) acts as
cholinesterase inhibitor, an enzyme targeted in the treatment of Alzheimer'disease
(Blom et al., 2006). The effects of this compound were comparable to galanthamine,
an approved drug for Alzheimer' disease. This discovery could lead to development of
drugs for neurological disorders and shows, that cyanobacteria are possible sources of
pharmaceuticals also for these diseases.
Figure 1-6: Nostocarboline from Nostoc 78-12A
During the last few years several novel and diverse metabolites combined
with relevant pharmaceutical activities (e.g. antibiotic, enzymes, antiviral, anticancer,
antifungal, and antiinflammatory agents as well as protease inhibitors) have been
discovered from cyanobacteria which clearly indicates that cyanobacteria have a
valuable potential for providing novel and diverse bioactive substances for drug
discovery and can be considered a prime source for leads for drugs; this has
stimulated researcher’s efforts to find novel and pharmacologically active
cyanobacterial metabolites (Jaspars & Lawton, 1998; Burja et al., 2001; Singh et al.,
2005; Sielff et al., 2006; Sivonen & Börner, 2008; Gademann & Portmann, 2008).

Chapter I Introduction
22
1.3 Aim of the work
Isolation and screening of cyanobacteria for antibiotics and other
pharmacologically active compounds have recently received considerable attention.
During the last decade a large number of novel bioactive molecules have been
isolated from cyanobacteria, but cyanobacteria are still viewed as unexplored source
of potential drugs (Sielaff et al., 2006). Especially the collections of cyanobacterial
strains from South East Asia where biodiversity is high (Tan, 2007) are still largely
unexplored.
In our going efforts toward finding novel and pharmacologically active
marine and terrestrial cyanobacterial metabolites, we have investigated 12 strains of
terrestrial cyanobacteria collected in Daklak province and one marine cyanobacterium
Lyngbya majuscula from Khanh Hoa province of Vietnam were studied with
following goals
1. Screening for antibacterial activity of the extracts prepared with organic
solvents of different polarity and water from 12 terrestrial cyanobacterial
strains;
2. Isolation, identification and structure elucidation of the antimicrobial
compounds from extracts with prominent activity;
3. Isolation, identification and structure elucidation of the cytotoxic
compounds from Lyngbya majuscula;
4. Culture optimization of selected strains showing strong antibacterial
activity to enhance biomass production and synthesis of active compounds.

Chapter II Materials and methods
23
2 Materials and methods
2.1 Biological materials
2.1.1 Soil cyanobacteria
The cyanobacterial strains were isolated from acidic soil samples collected
from rice, cotton and coffee fields in the Dak Lak province, Vietnam during April
2002-2003 and September 2002- 2003 (see fig. 2-1).
Figure 2-1: Map of Vietnam with the locality of 12 cyanobacterial strains in Dak Lak province
Dak Lak is a Central Highland province of Vietnam located at 11°44’-
13°25’N, 107°23’- 109°06’E (Ho, 2007).
The isolates were established as laboratory cultures at the Department for
Algal Biotechnology, Institute of Biotechnology (IBT), Hanoi, Vietnam. These strains
are maintained in the culture collection of the Institute of Pharmacy, Ernst-Moritz-
Arndt- University Greifswald, Germany as stock cultures.
Firstly, according to morphology and classification of Komárek &
Anagnostidis, 1989; 1999; 2005 and Anagnostidis & Komárek, 1990; Desikachary,

Chapter II Materials and methods
24
1959, and http://www.algaebase.org most of these strains were classified belonging to
the genera Anabaena, Nostoc, Calothrix, Oscillatoria, Scytonema and Westiellopsis.
Addtionally, genus Westiellopsis has been identified by molecular characterization of
16S rDNA sequence (Ho et al. 2005a) and four cyanobacterial strains which belong to
genus Calothrix were explored for the genetic relationships by using Randomly
Amplified Polymorphic DNA Polymerase Chain Reaction (RAPD-PCR) technique
(Ho et al., 2006).
Table 2-1: Cyanobacterial strains
Scientific
name
Strain
number
Morphological feature Classification
Oscillatoria sp.
TVN16
Trichomes long small without any
branching, not constricted at the cross-
walls, sheath absent; cells discoid, no
heterocysts in filament.
Oscillatoriaceae
Oscillatoriales
Anabaena sp.
TVN40
Trichomes constricted at the cross-
walls, look like string of beads; cells
barrel-shaped, mostly longer than
broad; no heterocysts in filament; no
branches.
Nostocaceae
Nostocales
Nostoc
spongiaeforme
Agardh ex
Born. et Flah.
TVN7
Filaments flexuous, loosely entangled;
sheath thin; cells cylindrical or oblong;
heterocysts subspherical; spores in long
chains.
Nostoc
coeruleum
Lyngbye ex
Born. et Flah.
TVN14
Filaments densely entangled, flexuous,
sheath mostly indistinct; trichomes 5.1-
6.8 µm broad; cells short, barrel-shaped;
heterocysts subspherical; spores not
known.
Nostoc sp.
TVN9
Filaments single, flexuous, entangled;
cells nearly spherical, no heterocysts in
filament, no branches.
Nostocaceae
Nostocales
Calothrix
javanica de
Filaments single, pale blue-green to
olive green, lamellated sheath,

Chapter II Materials and methods
25
Wilde
TVN1
gradually attenuated to a pointed apex;
trichomes 5.1-6 µm broad; heterocysts
basal, 4-5.1 µm broad, 4.8-6 µm in
length; spores single or two together,
about 8.5-9 µm broad, 6-10 µm long
Calothrix elenkinii
Kossinsk.
TVN202
Filaments 80-250 µm, united in tuff,
swollen at the base, 6-9 µm broad;
sheath close to the trichome, thin, not
lamellated, open at the ends; trichomes
at the base 5.1-6.8 µm broad, apical hair
not formed; cells quadratic or somewhat
shorter; heterocysts single, basal, 4.1-
6.8 µm broad
Calothrix sp. TVN20 Filaments single, unbranched;
heterocysts basal.
Calothrix
marchica var.
crassa Rao,
C.B.
TVN201 Filaments in groups, irregularly bent
and closely entangled, 8.5-13.6 µm
broad; sheath thin, firm; trichomes 8.5-
11.9 µm broad, constricted at the septa,
ends tapering but without a hair, end
cell with a rounded apex, sometimes
pointed cells quadratic, as well as
shorter or longer than broader;
heterocysts single, basal, spherical or
subspherical, 8.5 µm broad, 5.1 µm
long.
Rivulariaceae
Nostocales
Scytonema
millei Bornet
ex Born. et
Flah.
TVN12 Filaments interwoven, false branches
erect; sheath firm brownish; heterocysts
discoid
Scytonema
ocellatum
Lyngbye ex.
Born. et Flah.
TVN10 Filaments up to 3 mm long, 10.2-16.3
µm; false branched; sheath firm, often
lamellated; trichomes 6.8-8.5 µm broad;
cells shorter than broad or quadratic;
heterocysts subquadratic, size 6.8-6.8
µm
Scytonemataceae
Nostocales

Chapter II Materials and methods
26
Westiellopsis
sp.VN
TVN22 Filaments with true branching
filaments of two kinds, primary
filaments and secondary filaments.
Main filaments torulose, constricted at
the cross-walls, with short barrel-shaped
cells or longer than broad, 8.5 µm
broad, 10.2 µm long. Branch filaments
growing erect, generally thinner, not
constricted at the cross-walls, with
cylindrical cells, 5.1 µm broad, 10.2-12
µm long. Heterocysts rounded-
cylindrical in main and branch
filaments, 5.1-8.5 µm or 10.2 µm broad,
1.9-13.6 µm long. Pseudohormocysts
formed on terminal portions of
secondary filaments. Endospores single
in each cell of the pseudohormocysts,
8.5- 11.9 µm diameter
Stigonemataceae
Stigonematales
Oscillatoria sp. (x 100) Anabaena sp. (x 100)

Chapter II Materials and methods
27
Nostoc spongiaeforme Nostoc coeruleum
Agardh ex Born. et Flah. (x 600) Lyngbye ex Born. et Flah.(x 600)
Nostoc sp. (x 100) Calothrix javanica de Wilde (x 600)
Calothrix elenkinii Kossinsk (x 100) Calothrix sp. (x 600)

Chapter II Materials and methods
28
Calothrix marchica var. crassa Rao,C.B.
a. Root part (x 600); b. Trichom(x 120)
Scytomema millei Scytonema ocellatum
Bornet ex Born. et Flah. (x 600) Lyngbye ex. Born. et Flah. (x 600)
Westiellopsis sp.VN (x 600), (x 100), respectively
Figure 2-2: Morphology of 12 cyanobacterial strains

Chapter II Materials and methods
29
2.1.2 Marine cyanobacteria
Living specimens of the marine cyanobacterium Lyngbya majuscula were
collected at Hon Khoi locality in Khanh Hoa province, Vietnam on August 20, 2007
(see fig. 2-3). Khanh Hoa province is located at the South Central Coast of Vietnam.
Its geographical coordinates are 108°40’33" to 109°27’55" E and 11°42’50" to
12°52’15" N, the length of the coast lines about 300 km. Hon Khoi is an area of Ninh
Hoa district of Khanh Hoa, this area is situated at 12° 34′ 44″ N, 109° 13′ 49″ E
Figure 2-3: 1A. Map of Vietnam with the collection area (the arrow indicates the collection site) 1B. The collection place of Lyngbya majuscula in Khanh Hoa province indicated (Geological Map of the sea waters of the Institute of Oceanography, Nha Trang, Vietnam)
1A
1B

Chapter II Materials and methods
30
Samples of the marine cyanobacterium Lyngbya majuscula Harvey ex Gomont
(Oscillatoriaceae), growing on rocks, dead corals, and gravel in the lower intertidal to
subtidal zone of shores and exposed to calm to moderate wave action were collected
by hand from a water depth of 0.1-1m, placed into sample containers and shipped in
the laboratory within the day. In the laboratory, the samples were air-dried in low
natural light, lyophilized later, and stored at -200C until use. A voucher specimen is
available in the Department for Algal Biotechnology, Institute of Biotechnology
(IBT) of the Vietnam Academy of Science and Technology (VAST), Hanoi and the
Ernst-Moritz-Arndt- University Greifswald, Germany under the strain number
LMVN.
The sample (see fig. 2-4) was identified morphologically as Lyngbya
majuscula Harvey ex Gomont (Oscillatoriaceae) by algologist Pham Huu Tri, the
Oceanography Institute, Nha Trang and Dr. Dang Diem Hong, the Department for
Algal Biotechnology, Institute of Biotechnology (IBT) of the Vietnam Academy of
Science and Technology (VAST), Hanoi.
The thallus of marine cyanobacterium Lyngbya majuscula Harvey ex
Gomont (Oscillatoriaceae) from Hon Khoi locality in Khanh Hoa province expanded,
up to 3 cm long, dull blue-green to brown or yellowish-brown in color, with very long
and curved filaments, seldom only slightly coiled, the sheath colorless, lamellated, the
trichomes blue-green, not constricted at the cross-wall, not attenuated at both ends,
the calyptra absent.
a

Chapter II Materials and methods
31
b c
Figure 2-4: a and b Natural habit (took photograph during low tide) of Lyngbya majuscula c Microscopic view of filament (x 40) of Lyngbya majuscula
2.1.3 Bacteria, yeast, and cancer cell lines as test organisms
• Gram positive bacteria Staphylococcus aureus ATCC 6538, Bacillus subtilis
ATCC 6051
• Gram negative bacteria Escherichia coli ATCC 11229, Pseudomonas
aeruginosa ATCC 27853
• Yeast Candida maltosa SBUG 700
• Cancer cell lines: 5637 cell line: bladder cancer cell line (German Collection
of Microorganisms and Cell Cultures (DSMZ Braunschweig, Germany)
2.2 Chemicals
2.2.1 Cultivation of cyanobacteria
All media were prepared and autoclaved at 1210C for 20 min before use, but
preparation of BG 11 medium citric acid combined with ferric ammonium citrate had
to be autoclaved separately and after that added to the autoclaved medium.
BG-11 medium (Rippka et al., 1979)
Ingredient Stock solution (g/100 mL)
Nutrient solution (mL/L)
NaNO3 15.0 10 K2HPO4. x 3 H2O 0.4 10 MgSO4. x 7 H2O 0.75 10 CaCl2 x 2 H2O 0.36 10 Citric acid 0.06 10 Ferric ammonium citrate 0.06 10 EDTA (disodium magnesium salt) 0.01 10 Na2CO3 0.2 10 *[Trace metal mix A5+Co] 1 Distilled water 919

Chapter II Materials and methods
32
*[Trace metal mix A5+Co]
Ingredient Stock solution (g/L)
Nutrient solution (mL/L)
H3BO3 2.86 MnCl2 x 4H2O 1.81 ZnSO4. x 7H2O 0.222 CuSO4. x 5H2O 0.079 Na2MoO4. x 2H2O 0.390 Co (NO 3)2. x 6H2O 0.0494 Distilled water 1.0 L
1
Modified BG-110 medium (BG-11 without nitrate)
2.2.2 Cultivation of bacteria and yeast as test organisms
Standard II nutrient agar for microbiology (Merck, Darmstadt, Germany)
Composition:
-Peptone from meat 3.45 g
-Peptone from casein 3.45 g
-Sodium chloride 5.1 g
-Agar- agar 13.0 g
Suspend 25 g standard II nutrient agar in 1 liter of demineralized water by
heating in a boiling water bath or in a current of steam; autoclave (15 min at 1210C),
pH: 7.5±0.2 at 250C
2.2.3 General laboratory chemicals
Ampicillin Merck, Darmstadt, Germany
Gentamycinsulfat Biochrom AG, Berlin, Germany
Nystatin- Dihydrat Carl Roth GmbH & Co.KG,
Karlsruhe,Germany
NaCl Carl Roth GmbH & Co.KG,
Karlsruhe,Germany
Trifluroacetic acid (TFA) for spectroscopy Merck, Darmstadt, Germany
2.2.4 Chemical reagents
• Anisaldehyd- sulphuric acid (AS) reagent (Merck, Darmstadt, Germany):

Chapter II Materials and methods
33
Solution of 10 mL acetic acid containing 0.5 mL anisaldehyde was added to a
mixture of 5 mL sulphuric acid and 85 mL methanol (Houghton and Raman,
1998).
• Iodonitrotetrazolium chlorid (INT) (Sigma, Steinheim, Germany): 5 mg of
the INT is dissolved in 1 mL of ethanol 50%.
2.2.5 Fatty acid analysis
Ethanol Merck, Darmstadt, Germany
KOH VWR, Darmstadt, Germany
HCl Bayer, Leverkusen, Germany
Na2SO4 VWR, Darmstadt, Germany
Methoxyamine (MeOX) Sigma- Aldrich, Munich, Germany
N-methyl-N-
trimethylsilyltrifluoroacetamide (MSTFA)
CS Chromatography Service,
Langerwehe, Germany)
2.3 Solvents
Acetone Carl Roth GmbH & Co.KG, Karlsruhe, Germany
Acetic acid Carl Roth GmbH & Co.KG, Karlsruhe, Germany
Acetonitrile (HPLC gradient
grade)
Prolabo VWR International company, made in EC
or Carl Roth GmbH & Co.KG, Karlsruhe,
Germany or Fisher Scientific, Loughborough, UK
Dichloromethane Carl Roth GmbH & Co.KG, Karlsruhe, Germany
Deionized water
Ethanol Carl Roth GmbH & Co.KG, Karlsruhe, Germany
Ethyl acetate Carl Roth GmbH & Co.KG, Karlsruhe, Germany
Methanol Carl Roth GmbH & Co.KG, Karlsruhe, Germany
Methanol (HPLC gradient
grade)
Prolabo VWR International company, made in EC
or AppliChem, AppliChem GmbH, Damstadt,
Germany
n-Hexane Carl Roth GmbH & Co.KG, Karlsruhe, Germany
All solvents were distilled prior to use except for HPLC and spectral grade
solvents were used for spectroscopic measurements.

Chapter II Materials and methods
34
2.4 Equipment in generally company, town, country
2.4.1 Cultivation of cyanobacteria
- For stock culture: 100-150 mL Erlenmeyer flasks (VWR GmbH, Darmstadt,
Germany)
- For batch culture: 1.5 L Fehrnbach flasks (Merck, Darmstadt, Germany)
- Large scale cultivation:
45 liter-glass fermentor Self-construction,Ernst-Moritz-Arndt-university,
Greifwald, Germany
pH Redox- transducer (Ecoline
pH 170)
Wissenschaftlich – Technische Werkstätten GmbH,
Weilheim, Germany
Heater Tetra GmbH, Melle, Germany
Centrifuge (Rotanta 460R) Hettich Zentrifugen, Tuttlingen; Germany
Centrifuge Stratos D37520 Heraeus Instruments , Osterode, Germany
Lyophylizer Alpha 1-4 Christ GmbH, Berlin, Germany
Filter paper Ø 185 mm with 12-
25 µm pore size
Schleicher & Schüll MicroscienceGmbH, Dassel,
Germany
Deep Freezer GTL 2811WS Bauknecht, Berlin Germany
Autoclave Varioklav, H+P Labortechnik, Germany
Sterilizer Memmert, Schwabach, Germany
2.4.2 Extraction
Laboratory sea sand Merck, Darmstadt, Germany
Porcelain mortar and pestle
250 mL and 500 mL
Erlenmeyer flasks
Merck, Darmstadt, Germany
System of rotatory vacuum
evaporator: pump B-178,
vacuum controller B-721, Rota
vapor R-114, water bath B-480
Büchi Labortechnik AG, Flawil, CH, Büchi & Co,
Berlin, Germany
Stirrer, Heidolph MR 3000 Schwabach, Germany
Shaker Kika Labortecknik Staufen, Janke & Kunkel
GmbH & Co.KG, Germany
Banderlin Sonorex RK103H Bandelin electronic, Berlin, Germany

Chapter II Materials and methods
35
Ultrasonic cleaner
Centrifuge (Rotanta 460R) Hettich Zentrifugen, Tuttlingen; Germany
100 mL centrifuge tubes, round
bottom
Carl Roth GmbH & Co.KG, Karlsruhe, Germany
Lyophylizer Alpha 1-4 Christ Gefriertrocknungsanlagen, Osterode,
Germany
Filter paper Ø 185 mm with 12-
25 µm pore size
Schleicher & Schüll MicroscienceGmbH, Dassel,
Germany
Banderlin Sonorex RK103H
Ultrasonic cleaner
Banderlin electronic, Berlin, Germany
2.4.3 Isolation of secondary metabolites
2.4.3.1 Thin layer chromatography (TLC)
TLC sheets: Silica gel 60, F254,
20x20 cm
Merck, Darmstadt, Germany
TLC tank Desaga GmbH, Heidelberg, Germany
Capillaries Hirschmann Laborgeräte GmbH & Co. KG, Eberstadt,
Germany
UV lamp Desaga SARSTEDT-
GRUPPE HP-UVIS
Desaga GmbH, Heidelberg, Germany
UVPMultiDoc-It, Digital
Imaging System
Cambridge, UK
Thermoplate Desaga
SARSTEDT-GRUPPE
Desaga GmbH, Heidelberg, Germany
2.4.3.2 Preparative TLC
Preparative TLC plate: PLC Silica gel F254, 2mm, 20 x 20 cm (Merck,
Darmstadt, Germany)
2.4.3.3 Open column chromatography
Glass column: 60 x 1.2 cm,
50 x 4.0 cm; 50 x 2.0 cm;
Schott Duran, Mainz, Germany

Chapter II Materials and methods
36
35 x 2.5 cm; 35 x 1.2 cm (h x i.d.)
50 mL, 100 mL, 250 mL, 500
mL, and 1000 mL Erlenmeyer
flasks
Merck, Darmstadt, Germany
Test tubes (10 mL) Schott Duran, Mainz, Germany
Fraction Collector Model 2110 Bio-Rad Laboratories, Richmond, USA
Silica gel 60 ( 0.015-0.040 mm;
0.040-0.063 mm)
Merck, Darmstadt,Germany
Sephadex LH-20 Amersham Biosciences AB, Uppsala, Sweden
Silica gel 60RP-18 ( 0.040-0.063
mm)
Merck, Darmstadt,Germany
Pasteur pipettes, 150 mm, 230 mm VWR GmbH, Darmstadt, Germany
2.4.3.4 HPLC
Synergi Polar RP 4 µm (80 Å) column (Phenomenex Ltd, Aschaffenburg,
Germany)
+ Analytical column (250 x 4.6 mm)
+ Semi-preparative column (250x10 mm)
HPLC system (Kontron Instruments, Italy)
+ Diode array detector (DAD 440)
+ Autosampler 360 (SA360)
+ Pump 422 & 422 S
Water-system Clear UV plus SG Water Preparation and Recycling GmbH,
Germany
HPLC vials (1.5 mL) (VWR GmbH, Darmstadt,Germany)
2.4.4 Agar plate diffusion test
Petri dishes plastic or glass (Ø 90 mm) Merck, Darmstadt, Germany
Burner, beaker, variable Eppendorf
pipettes with sterile tips, tweezers
Sharp nails, polystyrene board,
inoculating loops, lineal
Steril antibiotic test paper discs Ø6mm Schleicher & Schuell Microscience

Chapter II Materials and methods
37
GmbH, Dassel, Germany
20-50 mL standard glasses Schott Duran, Mainz, Germany
Incubator Mytron BS 120 Memmert, Schwabach, Germany
Laminar flow box Heraeus Instruments, Hanau, Germany
2.4.5 Bioautographic TLC assay
Thin layer chromatography sheets
silica gel 60 F254, 20x20 cm
Merck KgaA, Darmstadt, Germany
Petri dishes 20- 50 mL (Ø 90 mm) Merck, Darmstadt. Germany
Incubator Mytron BS 120 Memmert, Schwabach, Germany
Sterile standard glass Merck, Darmstadt, Germany
Laminar flow box Heraeus Instruments, Hanau, Germany
2.4.6 Fatty acid analyses
GC/MS Firma Agilent (USA)
Gaschromatograph G1530N
MSD G2588A
Software G1701 CA
Syringe G2613 A
syringe
sample rack
column
Figure 2-5: Agilent 6890N gas chromatograph and mass selective detector (Agilent®5973 Network MSD)

Chapter II Materials and methods
38
2. 5 Cultivation of cyanobacteria
2.5.1 The stock culture
For maintenance of laboratory culture, 2- 3 mL of a 3 weeks old
cyanobacterial stock culture was used as inoculum in 50 mL of autoclaved BG 11
medium in 150 mL Erlenmeyer flasks. The cultivation was carried out at 20 ±20C,
under continuous illumination of 8µmol/m2 by cool fluorescence lamps. The stock
cultures were maintained for 20-30 days.
2.5.2 The batch culture
During this work 12 cyanobacterial strains (see 2.1.1) were cultured in batch
cultures. Aliquots of 50 mL from the stationary phase stock cultures were used to
inoculate 500 mL of autoclaved BG11 medium in 1.5 liter Fehrnbach flasks. These
samples were cultivated at 20 ±20C, under continuous illumination of 8µmol/m2 by
cool fluorescence lamps. The cyanobacterial cultures were harvested after 4-6 weeks.
The cells were separated from the medium by centrifugation (4000 rpm/ 10 min/
100C) followed by filtration with filter paper. The biomasses were lyophilized and
stored at -20°C until use while cultivation media were concentrated to 1/10 (v/v) by
rotary evaporation in vacuum at 400C and extracted immediately with EtOAc solvent.
2.5.3 The large scale culture
During this work the large scale cultivation was applied for 5 cyanobacterial
strains, Westiellopsis sp. VN, Anabaena sp., Nostoc sp., Scytonema millei, and
Calothrix elenkinii.
The large scale cultivation was carried out in a 45 liter-glass fermentor (Mundt
et al., 2001). The fermentor was cleaned by distilled water and 70% isopropanol
before use. At the beginning, the fermentor was filled with 15 L of medium and after
1- 2 hours 1.5 L of growing culture (after 20 days of cultivation in three Fehrnbach
flasks) was added. Afterwards, every day 5 L of medium were added into the
fermentor until 35 L of medium were reached. The cultures were illuminated
continuously with banks of cool white fluorescent tubes of 8µmol/m2 and incubated
at temperature of 26°C to 28°C adjusted using a heater. The pH-value of the large-
scale culture was adjusted to 7.4-8.5 using CO2 supplementation.
Strain Westiellopsis sp. VN was grown for 8 weeks at 28°C and pH of 7.4
Strain Anabaena sp. was grown for 6 weeks at 26°C and pH of 8.5

Chapter II Materials and methods
39
Strain Nostoc sp. was grown for 4 weeks at 26°C and pH of 8.5
Strain Scytonema millei was grown for 4 weeks at 26°C and pH of 8.5
Strain Calothrix elenkinii was grown for 7 weeks at 28°C and pH of 7.4
The biomass was collected by centrifugation at 6500 rpm in a refrigerated
continuous-flow centrifuge and lyophilized, then stored at -200C.
a
b c
Figure 2-6: Cultivation of cyanobactria: a. Stock culture;
b. Batch culture; c. Large scale culture
2.6 Extraction
2.6.1 Extraction of intracellular compounds
The harvested cells were freeze-dried and then successively extracted with
three different organic solvents which increasing polarity starting with n-hexane,
methanol, water in three steps (Mundt et al., 2001).
Firstly, 5 g freeze-dried biomass was crushed with 1g sea sand and 5 mL of
the first organic solvent (n-hexane) using porcelain mortar and pestle to get
homogenous suspension. The remaining 245 mL n-hexane was added to the cell
suspension followed by homogenization for 7 minutes in ultrasonic bath. After this,
the biomass was extracted for 1 hour under stirring at room temperature. The
supernatant was separated from the residue by centrifugation (Centrifuge 96R, 4500

Chapter II Materials and methods
40
rpm, 10 minutes, and 4°C). The residue was extracted with n-hexane three times in
all. After filtration the supernatants were pooled and evaporated to dryness with a
rotary evaporator. The residue of the biomass was dried at room temperature over
night. Subsequently, the dried residue was further extracted 3 times with the next
solvents (methanol followed by water).
The organic solvents were removed by rotary evaporation in vacuo at 400C
to get dry extracts. The water was reduced by rotary evaporation at 400C and removed
completely by lyophilization (lyophylizer Alpha 1-4). The dried extracts were
weighed and stored at -200C until use.
2.6.2 Extraction of extracellular compounds
For media extraction, 3L of cyanobacterial culture medium were
concentrated to 250-300 mL by rotary evaporation in vacuum at 400C. The media
were then shaken for 24 h with equal volume of ethyl acetate then separated by
separating funnel. Extraction of the lower phase (media) was repeated three times.
The upper phase was collected to get ethyl acetate extract. All ethyl acetate extracts
obtained from the three extractions were combined, dried with sodium sulfate and
reduced to dryness in vacuum. The dried extract was weighed and stored at -200C
until use.
Cyanobacte r ia
5g freeze-dried biomass Media
n- hexane ext ract
2 50m l n-hexane/ 3 t im es/ st ir ring
Residue
Methanol ext ract
2 50m l methanol/ 3 t im es/ st ir ring
Residue
W ater ext ract
2 5 0m l w ate r/ 3 t im es/ st ir ring
Residue
Ethyl acet ate ext ract
2 50m l ethyl aceta te/
3 t im es/ shaking
Bioassay
Suspension
- Crushed w ith n-hexane and see sand in mortar
-Ult rasonicated for 7min
Scheme 2-1: Scheme of extraction

Chapter II Materials and methods
41
2.7 Bioassays
2.7.1 Assays for antimicrobial activity
2.7.1.1 Agar diffusion assay
An agar diffusion assay according to the Pharmacopoea Europaea was used
to determine antibacterial and antifungal activity in screening the extracts (see 2.6)
and for evaluation the activity of fractions during separation process and isolated
compounds.
As test organisms were used the gram positive bacteria Staphylococcus
aureus ATCC 6538 and Bacillus subtilis ATCC 6051, the gram negative bacteria
Escherichia coli ATCC 11229 and Pseudomonas aeruginosa ATCC 27853 and yeast
Candida maltosa SBUG 700. All experiments were repeated 3 times. Inhibition zone
was measured including 6 mm paper disc.
Ampicillin (10 µg for Staphylococcus aureus and Bacillus subtilis; 50 µg
for Escherichia coli), Gentamycin (25 µg for Pseudomonas aeruginosa) or Nystatin
(5 µg for Candida maltosa) were used as positive control. The solvent was used as
negative control.
A sterile paper disc with a diameter of 6 mm was loaded with 50 µL test
solution. For screening 2 mg of cyanobacterial extracts dissolved in the extraction
solvent were applied on the paper disc. During bioassay-guided fractionation of
cyanobacterial extracts, the extract was tested in a concentration of 2.0 mg/disc,
fractions after first separation were tested in a concentration of 500 µg/disc and
fractions of second separation and pure compounds were tested in a concentration
between 100 and 200 µg/disc. One paper disc was loaded with only solvent as solvent
control (50 µL) and another was loaded with ampicillin, gentamycin or nystatin as
positive control. The paper discs were fixed by pins on a polystyrene plate and dried
for 2 hours under sterile conditions to eliminate solvents completely.
The bacteria were cultured on nutrient agar plate at 370C for 24 h (bacteria)
and at 28°C for 48 h (Candida maltosa) then maintained at 40C. For antibiotic test,
from the bacterial stock culture an inoculum was spread on a nutrient agar plate and
incubated at 370C for 24 h (bacteria) and at 28°C for 48 h (Candida maltosa) before
use. From this culture a pin-head size inoculum was suspended in 2 mL of sterile
0.9% NaCl and mixed thoroughly. 200 µL of this suspension was diluted with 20 mL
sterile warm agar medium and poured immediately into the Petri dish. Temperature of

Chapter II Materials and methods
42
nutrient agar should be below 400C. After solidification of the agar (about 15 minutes
under sterile conditions) the paper discs containing test samples were placed on the
surface of the agar and the Petri dishes were kept at 4°C over 4 h for prediffusion.
After this, the plates were incubated for 24 h at 37°C for bacteria and for 48 h at 28°C
for Candida maltosa in an inverseposition. At the end of the incubation period, the
inhibition zones were measured and expressed as the diameter of the clear zone
including the diameter of the paper disc (∅ 6 mm).
For better detection of inhibition zones the agar plates were sprayed with
INT solution. Inhibition zones were visible as clear zones around the paper discs
against a dark red background.
2.7.1.2 Bioautographic TLC assay
For bioautographic TLC, an amount of an extract or a fraction is put on a
TLC plate, and the plate is covered with a suspension of bacteria in agar. Incubation
permits growth of the bacteria. Zones of inhibition are then visualized using spray
reagents (Hamburger and Cordell, 1987).
500 µg of extract or fraction were applied on analytical TLC sheet (6,5x3
cm) in duplicate and developed in the same solvent system. The control
chromatogram was detected at 254 nm and 356 nm UV light and by spraying with
anisaldehyde/sulfuric acid reagent, Rf values were calculated. The test chromatogram
was dried under sterile conditions for 3 hours.
Approximately 15 mL of nutrient medium was poured into a Petri disc (Ø 90
mm) as basic layer. After solidification of the agar, the test chromatogram was
applied free of air bubbles on the surface of nutrient agar. 20 mL of nutrient medium
inoculated with 200 µL of bacterial suspension containing S. aureus as described in
2.7.1.1 was poured over the test chromatogram as top layer. The Petri dish was
incubated at 4°C for 3 h and afterwards incubated at 37°C as described for agar
diffusion assay. The inhibition zones were detected with INT reagent. After spraying
with INT, the inhibition zones appear as clear sports against the red background. The
inhibition zones were compared with the Rf values of the control chromatogram so
that the active compounds were located on TLC.

Chapter II Materials and methods
43
2.7. 2 Assays for cytotoxic activity
Cytotoxicity data of extracts, fractions, and pure compounds of Lyngbya
majuscula towards 5637 cell line [bladder cancer cell line {(German Collection of
Micro organisms and Cell Cultures (DSMZ Braunschweig, Germany)} were provided
by Dr. Wende, Department of Phamaceutical Biology, Institute of Pharmacy, Ernst-
Moritz-Arndt-University of Greifswald, Germany. Method was modified according to
Bracht and Bednarski (Bracht et al., 2006). Test was performed as previously
published (Bui et al., 2007).
2.8 Fractionation and isolation of the secondary metabolites of 6
cyanobacterial strains
2.8.1 Fractionation and isolation of the secondary metabolites of Westiellopsis
sp.VN
The active methanol extract obtained from extraction of lyophilized
biomass from large scale culture of this strain was separated first by silica gel
chromatography, followed by gel filtration, and finally reversed-phase HPLC (see
scheme 2-2).
The methanol extract (260 mg) obtained from 2 g of the lyophilized
biomass was fractionated using silica gel column chromatography. For preparation of
the column 30 g of silica gel 60 (0.015-0.040 mm) was mixed and saturated with the
first solvent system for 1 hour at room temperature. This silica gel solution was then
poured into the column (35 x 2.5 cm, h x i.d.) The column was rinsed with the first
solvent system for 30 minutes and a thin layer (1cm) of sea sand was applied on the
top of column. The methanol extract was dissolved in a small amount of the first
solvent system and applied on the surface of silica gel bed. The column was then
eluted with 300 mL of each mobile phase, first DCM, followed by DCM/EtOAc
(95:5), DCM/EtOAc (90:10), DCM/EtOAc (50:50), EtOAc, EtOAc/MeOH (75:25),
EtOAc/MeOH (50:50), and finally MeOH. Each eluting solvent was collected in a
different flask at flow rate 0.4 mL/min. After that, eight major fractions (FI to FVIII)
were obtained, and solvents were removed in vacuo using a rotary evaporator. All
fractions were tested for their antibacterial activity. The fractions FI, FII, FIII eluting
with 100% DCM, 95% DCM/EtOAc, and 90% DCM/EtOAc were pooled since they
all exhibited approximately the same antibacterial activity.

Chapter II Materials and methods
44
The pooled fractions (68.5 mg) were further seperated using sephadex LH-
20 column chromatography. An amount of 18 g LH-20 gel was swollen in 100 mL
H2O/MeOH (10:90) for 1 hour at room temperature. The suspension was then poured
slowly into the column (60 x 1.2 cm, h x i.d.) and rinsed with H2O/MeOH (10:90)
until the stationary was stable and reached a height of 50 cm. Then, sample dissolved
in a small amount of the first solvent system was softly applied onto the column
eluting first with 200 mL H2O/MeOH (10:90) and followed by 150mL MeOH, and
finally washing with 100mL H2O/Aceton (50:50). The outflow of the column was
collected in sub-fractions of 5mL automatically by fraction collector with a flow rate
0.3 mL/min. The sub-fractions were analyzed by TLC using n-hexane/EtOAc/MeOH
(75:25:5). TLC plate was visualized under UV light at 254 and 366 nm and by use of
anisaldehyde/sulfuric acid reagent. Sub-fractions with the same spots on TLC were
combined to main fractions. After TLC analyzing, four major fractions (WF1 to WF4)
were collected and tested for antibacterial activity. Because fraction WF1 which eluted
with 10% H2O/MeOH exhibited the highest antibacterial activity, fraction WF1 was
further purified.
The fraction WF1 was further purified by using a semi-preparative HPLC
column Synergi POLAR-RP 80A (250×10mm, 4 micron) with a flow rate of 3.0
mL/min and detection at 210, 220, 238, 254 and 366 nm. A concentration of 500
µg/50 µL was injected per run. Altogether, 50.0 mg were purified using the step
gradient described in table 2-2. Nine fractions (WF1-1-WF1-9) were collected under
UV of 238nm and then tested for the activity against S .aureus. Fractions WF1-3, WF1-
5, WF1-6, and WF1-8 exhibited significant antibacterial activity were therefore used for
structural elucidation.
Table 2-2: Step gradient used in purification of fraction WF1 by semi-preparative HPLC
Time (min) 0.50 3.50 40.50 42.50 50.50 52.50
Solvent A (%) 80 50 15 0.0 0.0 80.0
Solvent B (%) 20 50 85 100 100 20
Solvent A: H2O and solvent B: CH3CN; Flow rate: 3.0 mL/min; HPLC column: Synergi polar-
RP80A/250×10mm, 4 micron

Chapter II Materials and methods
45
3 x MeOH
WF1-1 WF1-2 WF1-3 WF1-4 WF1-5 WF1-6 WF1-7 WF1-8 WF1-9
WF1 WF2 WF3 WF4
LH-20 column
H2O/MeOH (1:9), MeOH, H2O/Aceton (1:1)
3 x n-Hexane
3 x EtOAc
Lyophilized biomass
n-Hexane ext.Residue
EtOAc ext. Residue
MeOH ext.
FI FII FIII FIV FV FVI FVII FVIII
Silica gel column
DCM/ EtOAc/ MeOH gradient
-Semi-preparative HPLC Synergi-Polar RP-CH3CH/H2O gradient
Structure elucidation
Scheme 2- 2: Extraction, fractionation, and isolation of the secondary metabolites of Westiellopsis
sp.VN
2.8.2 Fractionation and isolation of the secondary metabolites of Calothrix
javanica
The active methanol extract obtained from extraction of lyophilized
biomass from batch culture of this strain was subjected to RP C18 column
chromatography, followed by reversed-phase HPLC (see scheme 2-3).
210 mg of MeOH extract obtained from 1.25 g lyophilized biomass was
separated by reverse-phase C18 column chromatography. For preparation of the
column 15 g reverse-phase C18 silica gel was swollen in 50mL MeOH/EtOH/H2O
(45:45:10) for 3 hours at room temperature. The slurry was poured into the column
(35 x 1.2 cm, h x i.d.) and column was then rinsed with initial mobile phase for 30
minutes. After that, the sample was dissolved in a 1mL of initial mobile phase and
applied onto the surface of the gel bed. The column was initially eluted with 200 mL
MeOH/EtOH/H2O (45: 45:10), followed by 200 mL MeOH/H2O (90:10), 200 mL
MeOH, 200 mL MeOH/Acetone (50:50), and finally 200 mL DCM. The outflow of
the column was collected in sub-fractions of 4 mL automatically by fraction collector
at flow rate 0.3 mL/min. The received sub-fractions were analyzed by TLC in the first
solvent system, and then detected under UV light at 254 nm and 366 nm and by
spraying with AS reagent and heating. Sub-fractions with the same spots on TLC
were combined to main fractions. After TLC analyzing ten major fractions (CJFI to

Chapter II Materials and methods
46
CJFX) were received and tested for the antibacterial activity. The fraction CJFII eluted
with MeOH/EtOH/H2O (45:45:10) was further purified because this fraction showed
antibacterial activity.
The purification of fraction CJFII was carried out using a semi-preparative
HPLC column Synergi POLAR-RP 80A (250×10mm, 4 micron) with a flow rate of
3.0 mL/min and detection at 210, 220, 238, 246, 254 and 366 nm. A concentration of
2 mg/50 µL was injected per run. Altogether, 86.0 mg of CJFII were purified using the
step gradient described in table 2-3. Seven peaks were collected separately at 220 nm
to yield 7 fractions. Among of them, the fraction CJFII-4 was the most pure, this
fraction was therefore used for structural elucidation.
Table 2-3: Step gradient used in purification of fraction CJFII by semi-preparative HPLC
Time (min) 0.50 16.50 24.50 30.50 31.50
Solvent A (%) 30 15 0 0 30
Solvent B (%) 70 85 100 100 70
Solvent A: H2O+0.05%TFA and solvent B: MeOH; Flow rate: 3.0 mL/min; HPLC column: Synergi
polar-RP80A/250×10 mm, 4 micron
3 x n-Hexane
Lyophilized biomass
n-Hexane ext.3 x MeOH
Residue
MeOH ext.
Silica gel 60 RP18 column
- MeOH/ EtOH/H2O= 45:45:10
- MeOH/ H2O= 90:10
- MeOH
- MeOH/ Acetone= 1:1
- DCM
- Semi-preparative HPLC Synergi Polar RP- MeOH/(H2O+0.05%TFA) gradient
CJFI CJFII CJFIII CJFIV CJFV CJFVI CJFVII CJFVIII CJFIX CJFX
CJFII-1 CJFII-2 CJFII-3 CJFII-4 CJFII-5 CJFII-6 CJFII-7
Structure elucidation
H2O ext.
Residue3 x H2 O
Scheme 2-3: Extraction, fractionation, and isolation of the secondary metabolites of Calothrix javanica

Chapter II Materials and methods
47
2.8.3. Fractionation and isolation of the secondary metabolites of Scytonema
ocellatum
The active methanol extract obtained from extraction of lyophilized
biomass from batch culture of this strain was first fractionated using RP C18 column
chromatography, followed by silica gel column chromatography, and finally reversed-
phase HPLC (see scheme 2-4).
230 mg of MeOH extract obtained from 1.26 g lyophilized biomass were
fractionated by RP C18 column chromatography. An amount of 15 g reverse-phase C18
silica gel was swollen in 50mL MeOH/H2O (1:1) for 3 hours at room temperature.
The slurry was poured into the column (35 x 1.2 cm, h x i.d.). Column was then rinsed
with initial mobile phase for 30 minutes. After that, the sample was dissolved in 1 mL
of initial mobile phase and applied onto the surface of the gel bed. The elution was
carried out first with 200 mL MeOH/EtOH/H2O (45:45:10), 200 mL MeOH, and 200
mL MeOH/Acetone (10:10). The outflow of the column was collected in sub-fractions
of 5 mL automatically by fraction collector at flow rate 0.2 mL/min. The received
sub-fractions were analyzed by TLC in the first solvent system, and then detected
under UV light at 254 and 366 nm and by spraying with of AS reagent and heating.
Sub-fractions with the same spots on TLC were combined to main fractions. After
TLC analyzing 8 major fractions (SOFI to SOFVIII) were obtained and tested for the
antibacterial activity. Because only fraction SOFII which eluted with
MeOH/EtOH/H2O (45:45:10) exhibited strong antibacterial activity, this fraction was
further separated.
74.3 mg of fraction SOFII were separated using silica gel column. 10 g silica
gel (0.040-063 mm) was mixed with the first solvent system, n-hexane/EtOAc
(25:75), to form loose slurry for 30 minutes at room temperature. This slurry was then
poured into the column (35 x 1.2 cm, h x i.d.) and column equilibration was carried
out with the first solvent system for 30 minutes. The sample was dissolved in initial
mobile phase and applied onto the surface of the gel bed protected by a thin layer (1
cm) of sea sand. The column was eluted with 100 mL n-hexane/EtOAc (25:75), 60
mL EtOAc, 60 mL EtOAc/MeOH (50:50), and 100 mL MeOH. The outflow of the
column was collected in sub-fractions of 4 mL automatically by fraction collector at
flow rate 0.2 mL/min. The sub-fractions were analyzed by TLC in mobile phase
MeOH/EtOH/H2O (45:45:10). The thin layer chromatogram was detected under UV
light at 254 and 366 nm and by spraying with AS reagent and heating. Sub-fractions

Chapter II Materials and methods
48
with the same spots on TLC were combined to main fraction. After TLC analyzing
ten major fractions (SOFII-1 to SOFII-10) were collected and tested for the antibacterial
activity. The fractions SOFII-5 and SOFII-6 eluting with 50% EtOAc/MeOH exhibited
approximately the same antibacterial activity. Thus, these two fractions were pooled
and further purified.
The purification of pooled fractions was carried out using semi-preparative
HPLC column Synergi POLAR-RP 80A (250×10mm, 4 micron) with a flow rate of
3.0 mL/min and detection at 210, 220, 238, 246, 254 and 366 nm. A concentration of
500 µg/50 µL was injected per run. Altogether, 26.0 mg of the mixture were purified
using the step gradient described in table 2-4. Three peaks were collected separately
under UV of 220 nm to give three fractions. All these fractions were tested for
antibacterial activity. Because the fraction FSO3 exhibited antibacterial activity, this
fraction was used for structural elucidation.
Table 2-4: Step gradient used in purification the pooled fractions (SOFII-5, SOFII-6) by semi-preparative HPLC
Time (min) 0.50 18.50 21.50 24.50 25.50
Solvent A (%) 95 5 0 0 95
Solvent B (%) 5 95 100 100 5
Solvent A: H2O and solvent B: CH3CN; Flow rate: 3.0 mL/min; HPLC column: Synergi Polar-
RP80A/250×10mm, 4 micron
Lyophilized biomass
Residue
Silica gel 60 RP18 column
- MeOH/ EtOH/H2O= 45:45:10
- MeOH
- MeOH/Acetone= 1:1
SOFI SOFII SOFIII SOFIV SOFV SOFVI SOFVII SOFVIII
Silica gel column
- n-hexane/EtOAc =25:75
- EtOAc
- EtOAc/MeOH =50:50
- MeOH
SOFII-1 SOFII-2 SOFII-3 SOFII-4 SOFII-5 SOFII-6 SOFII-7 SOFII-8 SOFII-9 SOFII-10
- Semi-preparative HPLC Synergi Polar RP
- CH3CN/H2O gradient
FSO1 FSO2 FSO3
Structure elucidation
Residue
3 x n-Hexane
n-Hexane ext.
3 x H2OMeOH ext.
3 x MeOH
H2O ext.
Scheme 2-4: Extraction, fractionation, and isolation of the secondary metabolites of Scytonema ocellatum

Chapter II Materials and methods
49
2.8.4 Fractionation and isolation of the secondary metabolites of Anabaena sp.
The ethyl acetate extract obtained from the microscopically cell-free growth
medium of Anabaena sp. of the large scale culture exhibiting very strong antibacterial
activity against Gram-positive and Gram-negative bacteria, as well as yeast Candida
maltosa was analyzed by HPLC. Based on the results of the HPLC analysis, the ethyl
acetate extract was separated by semi-preparative HPLC. Procedure of this separation
was carried out by using a semi-preparative HPLC column Synergi POLAR-RP 80A
(250×10mm, 4 micron) with a flow rate of 3.0 mL/min and detection at 210, 220, 238,
254 and 366 nm. A concentration of 1mg/50 µL was injected per run. Altogether, 12.0
mg of the crude ethyl acetate extract obtained from 4L culture medium were purified
using the step gradient described in table 2-5. Peaks were collected at 238 nm. Seven
fractions were obtained from ethyl acetate extract by semi-preparative HPLC and
tested for antimicrobial activity. Since only fraction AF6 exhibited activity against
different bacteria (Gram-positive and Gram-negative) and the yeast Candida maltosa,
this fraction was used for structure elucidation (see scheme 2-5).
Table 2-5: Step gradient used for purification of the EtOAc extract by semi-preparative HPLC
Time (min) 0.50 12.50 18.50 22.50 24.50 26.50
Solvent A (%) 95 75 40 0.0 0.0 80.0
Solvent B (%) 5 25 60 100 100 5
Solvent A: H2O and solvent B: CH3CN; Flow rate: 3.0 mL/min; HPLC column: Synergi polar-
RP80A/250×10mm, 4 micron
Grow th medium
- Semi- preparative HPLC Synergi Polar RP- CH3CN/ H2O gradient
Structure elucidat ion
250ml ethyl acetate/ 3times/ shaking/ 24h
at room temparature
Ethyl acetate ext .
First AFI AFII AFIII AFIV AFV AFVI
Scheme 2-5: Extraction, fractionation, and isolation of the secondary metabolites of Anabaena sp.

Chapter II Materials and methods
50
2.8.5 Fractionation and isolation of the secondary metabolites of Nostoc sp.
The methanol extract obtained from extraction of lyophilized biomass from
large scale culture of this strain was subjected to silica gel column chromatography,
followed by RP C18 column chromatography, and reversed-phase HPLC (see scheme
2-6).
760 mg of MeOH extract obtained from 3.75 g lyophilized biomass were
fractionated using silica gel column. 60 g silica gel (0.040-063 mm) were suspended
and saturated in first solvent n-hexane/EtOAc (90:10) for 1 hour at room temperature.
This silica gel solution was then poured carefully into the column (60 x 2.5 cm, h x
i.d.). The column was equilibrated with first solvent for 1 hour. The extract was
previously suspended in a small amount of methanol and carefully applied on the
surface of the column protected by a thin layer of sea sand. The column was eluted
with 450 mL of each mobile phase with increasing polarity starting with n-
hexane/EtOAc (90:10), and followed by n-hexane/EtOAc (40:60), n-hexane/EtOAc
(20:80), MeOH, and MeOH/H2O (95:5). Each eluting solvent was collected in a
different flask. After that 5 fractions (NFI to NFV) were obtained and tested for
antibacterial activity. Because fraction NFIV which eluted with MeOH exhibited the
strongest antibacterial activity, fraction NFIV was further separated.
360 mg of fraction NFIV was separated by RP C18 column chromatography.
25 g reverse-phase C18 silica gel was swollen in 50ml of initial mobile phase
MeOH/H2O (9:1) for 3 hours at room temperature. The slurry was poured into the
column (35 x 2.5 cm, h x i.d.). Column was then rinsed with initial mobile phase for
30 minutes. Amount of 360 mg fraction NFIV was dissolved in a minimum of initial
mobile phase and applied on the surface of column. The elution was carried out first
with 300 mL MeOH/H2O (90:10), 300 mL MeOH, and finally with 300 mL DCM.
The outflow of the column was collected in different flasks at flow rate 0.4 mL/min.
After that 3 major fractions (NFIV-1 to NFIV-3) were obtained and tested for the
antibacterial activity. Because fraction NFIV-1 which eluted with MeOH/H2O (90:10)
exhibited the strongest antibacterial activity, this fraction was further separated.
The purification of fraction NFIV-1 was carried out using the semi-
preparative HPLC column Synergi POLAR-RP 80A (250×10mm, 4 micron) with a
flow rate of 3.0 mL/min and detection at 210, 220, 238, 246, 254 and 366 nm. A
concentration of 1 mg/50 µL was injected per run. Altogether, 150.0 mg of NFIV-1
were purified using the step gradient described in table 2-6 and the purification was

Chapter II Materials and methods
51
monitored by diode array detector (DAD) at wavelength 238 nm. After that, four
fractions were collected, among of them, fraction NsF2 exhibited significant
antibacterial activity. Thus, fraction NsF2 was used for structure elucidation.
Table 2-6: Step gradient used for purification of fraction NFIV-1 by semi-preparative HPLC
Time (min) 0.50 8.50 12.50 16.50 18.50
Solvent A (%) 60 15 0 0 60
Solvent B (%) 40 85 100 100 40
Solvent A: H2O+0.05%TFA and solvent B: MeOH; Flow rate: 3.0 mL/min; HPLC column: Synergi
polar-RP80A/250×10mm, 4 micron
Lyophilized biomass
Residue
NFI NFII NFIII NFIV NFV
NFIV-1 NFIV-2 NFIV-3
- Semi-preparative HPLC Synergi Polar RP
- MeOH/(H2O+0.05%TFA) gradient
NsF1 NsF2 NsF3 NsF4
Structure elucidation
Residue
3 x n-Hexane
n-Hexane ext.
3 x H2OMeOH ext.
3 x MeOH
H2O ext.
Silica gel column
n-hexane/EtoAc/MeOH/H2O
gradient
Silica gel 60 RP18 column-MeOH/H2O=90:10
-MeOH
-DCM
Scheme 2-6: Extraction, fractionation, and isolation of the secondary metabolites of Nostoc sp.
2.8.6 Fractionation and isolation of the secondary metabolites of Lyngbya
majuscula
2.8.6.1 Method 1
The active MeOH extract obtained from lyophilized biomass was subjected
initially to repeated silica gel column chromatography, and finally separated by
preparative TLC (see scheme 2-7).
The methanol extract (620 mg) obtained from 30 g lyophilized biomass was
fractionated on silica gel column. For preparation of the column 150 g of silica gel 60
(0.040-063 mm) were suspended and saturated in first solvent n-hexane/EtOAc

Chapter II Materials and methods
52
(60:40) for one hour at room temperature. This silica gel solution was then poured
into the glass column (50 x 4.0 cm, h x i.d.) until the gel bed reached a height of 40
cm. After that, the silica gel column was flushed only with first solvent n-
hexane/EtOAc (60:40) for 30 minutes for stabilizing. The methanol extract was
dissolved in 1mL MeOH and applied softly on the surface of silica gel bed protected
by a thin layer (1 cm) of sea sand, and elution was initiated with 500 mL n-
hexane/EtOAc (60:40), followed by 500 mL n-hexane/EtOAc (40:60), 400 mL
EtOAc, 400 mL EtOAc/MeOH (60:40), 400 mL EtOAc/ MeOH (40:60), and 400 mL
MeOH. The outflow of the column was collected in sub-fractions of 3-7 mL by
fraction collector at flow rate 0.3 mL/min. Fifteen major fractions (F1 to F15) were
collected according to the bands detected on TLC developed by using n-
hexane/EtOAc/MeOH/CH3COOH (75:25:5:3), then detected under UV light at 254
and 366 nm or by spraying with anisaldehyde/sulfuric acid reagent and heating. Based
on the results of TLC analysis, 7 fractions were chosen for testing cytotoxic activity.
The fraction F8 eluting with 100% EtOAc exhibited the strong activity and only one
main spot in TLC. Thus, further separation of this fraction was necessary.
A portion of the fraction F8 (90 mg) was further separated on silica gel
column. Amount of 120 g silica gel 60 (0.040-063 mm) was saturated in the first
mobile phase n-hexane/EtOAc (40:60) and poured into the column (50 cm x 4.0 cm, h
x i.d.). The column was rinsed with the first mobile phase for 30 minutes. After that,
90 mg of fraction F8 was dissolved in 2 mL of the first mobile phase and loaded on
the surface of the silica gel protected by a thin layer (1 cm) of sea sand. 450 mL n-
hexane/EtOAc (40:60), 450 mL n-hexane/EtOAc (10:90), and 400 mL MeOH were
respectively used to elute substances of fraction F8 from the column. The outflow of
the column was collected in sub-fractions of 4 mL automatically by fraction collector
at flow rate of 0.4 mL/min. Six major fractions (F8-1 to F8-6) were obtained
according the TLC pattern developed in n-hexane/EtOAc/MeOH/CH3COOH
(75:25:5:3), then detected under UV light at 254 and 366 nm or by spraying with
anisaldehyde/sulfuric acid reagent and heating. All fractions (F8-1 to F8-6) were tested
for cytotoxic activity. Because fraction F8-3 which eluted with 10% n-hexane/EtOAc
exhibited a strong cytotoxic activity, this fraction was further purified.
A portion of the fraction F8-3 (30.2 mg) was applied onto a preparative TLC
plate (Merck, Silica gel F254, thickness 0.25 mm). The chromatogram was developed
with mobile phase n-hexane/EtOAc/MeOH/CH3COOH (75:25:5:3) and five spots

Chapter II Materials and methods
53
with Rf 0.28, 0.22, 0.18, 0.12, and 0.045 were scratched out from plate and resolved in
EtOAc by shaking for 3 x 15 minutes. The solutions were centrifuged, filtered and
evaporated under reduced pressure. After that, five fractions (F8-3-1 to F8-3-5) were
obtained. These five fractions (F8-3-1 to F8-3-5) were examined by analytical TLC and
HPLC. HPLC analysis showed that fraction F8-3-2 showed only one peak at Rf 0.22,
therefore structure elucidation of this fraction was done.
MeOH ext.
3 x n-Hexane
Lyophilized biomass
n-Hexane ext.3 x MeOH
Residue
H2O ext.
Residue
3 x H2O
Silica gel column7 solvent system
(n-hexane/EtOAc/MeOH gradient)
F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 F13 F14 F15
F8-1 F8-2 F8-3 F8-4
PTLC
n-hexane/EtOAc/MeOH/CH3COOH
75 : 25 : 5 : 3
F8-3-1 F8-3- 2 F8-3-3 F8-3-4 F8-3-5
Structure elucidation
Silica gel column-n-hexane/EtOAc=4:6
-n-hexane/EtOAc=1:9-MeOH
Scheme 2-7: Extraction, fractionation, and isolation of the secondary metabolites of Lyngbya majuscula (method 1)
2.8.6.2 Method 2
The active MeOH extract which obtained from lyophilized biomass of this
strain was subjected to silica gel column chromatography followed by reversed-phase
HPLC (see scheme 2-8).
240 mg of the methanol extract obtained from 15 g lyophilized biomass
were separated on silica gel column. For preparation of the column an amount of 45
g silica gel 60 (0.040-063 mm) was mixed and saturated in the first solvent system for
30 minutes at room temperature. This silica gel solution was then poured into the
column (50 x 2.0 cm, h x i.d) and the column was equilibrated by rinsing with first

Chapter II Materials and methods
54
solvent system for 30 minutes. 240 mg of the methanol extract were dissolved in 1
mL initial mobile phase and applied carefully on the surface of silica gel bed
protected by a thin layer of sea sand. Elution started initially with 450 mL n-
hexane/EtOAc (75:25), followed by 250 mL n-hexane/EtOAc (50:50), 250 mL n-
hexane/EtOAc (25:75), 150 mL EtOAc, 150mL EtOAc/MeOH (90:10), 150 mL
EtOAc/MeOH (25:75), 150 mL EtOAc/MeOH (50:50), 150 mL EtOAc/MeOH
(25:75), and finally 150 mL MeOH. The outflow of the column was collected in sub-
fractions of 3-7 mL at flow rate 0.3 mL/min by fraction collector except the outflow
of the column eluting with 25% EtOAc/MeOH to 100% MeOH, this was collected in
different flashes. The sub-fractions were analyzed and combined to main fractions by
TLC with n-hexane/EtOAc/MeOH/CH3COOH (75:25:5:3) as mobile phase, then
detected under UV light at 254 and 366 nm and by spraying with
anisaldehyde/sulfuric acid reagent and heating. After that, twenty two major fractions
(F1 to F22) were obtained. Based on the results of TLC analysis, three fractions F9, F10,
and F17 were chosen for testing cytotoxic activity. The fraction F10 eluting with 25%
n-hexane/EtOAc exhibited strong cytotoxic activity, thus, this fraction was further
separated.
The purification of fraction F10 was carried out using a semi-preparative
HPLC column Synergi POLAR-RP 80A (250×10mm, 4 micron) with a flow rate of
3.0 mL/min and detection at 210, 220, 238, 254 and 366 nm. A concentration of 500
µg/50 µL was injected per run. Altogether, 14.0 mg F10 were purified using the step
gradient described in table 2-7. Five fractions were collected at 210 nm and tested for
cytotoxic activity. The fractions F10-3 and F10-5 exhibited significant cytotoxic activity
and were therefore used for structural elucidation.
Table 2-7: Step gradient used in purification of fraction F10 by semi-preparative HPLC
Time (min) 0.50 3.50 14.50 24.50 34.50 39.50 49.50 52.5
Solvent A (%) 80 50 35 25 15 0.0 0.0 80
Solvent B (%) 20 50 65 75 85 100 100 20
Solvent A: H2O and solvent B: CH3CN; Flow rate: 3.0 mL/min; HPLC column: Synergi Polar-RP
80A/250×10mm, 4 micron

Chapter II Materials and methods
55
3 x n-Hexane
3 x EtOAc
Lyophilized biomass
n-Hexane ext.
Residue
EtOAc ext.
3 x MeOH
Residue
MeOH ext.
Silica gel columnn-Hexane/EtOAc/MeOH gradient
F10-1 F10-2 F10-3 F10-4 F10-5
F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 F13 F14 F15 F16 F17 F18 F19 F20 F21 F22
Structure elucidation
-Semi-preparative HPLC Synergi Polar RP
-CH3CN/H2O gradient
Scheme 2-8: Extraction, fractionation, and isolation of the secondary metabolites of Lyngbya majuscula (method2)
2.9 Structure elucidation of the isolated secondary metabolites
Nuclear magnetic resonance spectroscopic and high resolution-mass
spectrometric data were recorded in the Helmholtz Centre for Infection Research,
Braunschweig and Dr. Victor Wray and Dr. Rolf Jansen both the Helmholtz Centre
for Infection Research, Braunschweig provided expert help with the structure
elucidation of the secondary metabolites.
2.9.1 Structure elucidation of compounds isolated from Westiellopsis sp.VN and
Lyngbya majuscula
Mass spectrometry: HR-ES-IMS (positive ion mode) were recorded on a high
resolution Bruker MaXis mass spectrometer.
NMR spectroscopy: 1H, 13C NMR spectra were recorded at 300 K on a
Bruker AVANCE DMX 600 NMR spectrometer locked to the deuterium resonance of
the solvent, CDCl3. Chemical shifts are reference to the residual proton signal of the
solvent.

Chapter II Materials and methods
56
2.9.2 Structure elucidation of compounds isolated from Calothrix javanica and
Scytonema ocellatum
Mass spectrometry: HR-ESI-MS (positive ion mode) were recorded on a
Thermo Science LTQ Orbitrap mass spectrometer.
NMR spectroscopy: 1D (1H) and 2D (COSY, TOCSY, NOESY, HMQC
and HMBC) NMR spectra were recorded at 300 K on a Bruker AVANCE DMX 600
NMR spectrometer locked to the deuterium resonance of the solvent, trifluoroethanol-
d2/H2O (1:1). Chemical shifts are reference to the residual proton signal of the solvent
(1H: 3.95 and 13C: 60.85 ppm).
2.9.3 Structure elucidation of compounds isolated from Anabaena sp.
Mass spectrometry: HR-ESI-MS (positive ion mode) were recorded on a
Thermo Science LTQ Orbitrap mass spectrometer.
NMR spectroscopy: 1D (1H, 13C and DEPT-135) and 2D (COSY, HMQC
and HMBC) NMR spectra were recorded at 300 K on a Bruker AVANCE DMX 600
NMR spectrometer locked to the deuterium resonance of the solvent, trifluoroethanol-
d2/H2O (1:1). Chemical shifts are reference to the residual proton signal of the
solvent (1H: 3.95 and 13C: 60.85 ppm).
2.9.4 Structure elucidation of compounds isolated from Nostoc sp.
Mass spectrometry: ESI-MS were recorded on a Micromass Q-Tof-2 mass
spectrometer.
2.10 Investigation of the active ethyl acetate extract of Westiellopsis
sp. VN growth medium
According to data obtained from screening, the ethyl acetate extract from
growth medium of this cyanobacterial strain showed a strong antibacterial activity
against Gram-positive bacteria B. subtilis, S. aureus and Gram-negative bacteria E.
coli, P. aeruginosa as well as yeast Candida maltosa SBUG 700 (IZ of 13.5 mm).
This extract was therefore highly appropriate for investigation of antimicrobial
compounds.
Bioautographic assay was used to locate the active principles of ethyl acetate
extract from growth medium of Westiellopsis sp.VN on TLC chromatogram (see

Chapter II Materials and methods
57
2.7.1.2.). The extract was loaded on silica gel TLC plate and developed with three
mobile phases:
SS1 (n-hexane/ EtOAc/MeOH= 75:25:5)
SS2 (EtOAc/MeOH/H2O= 100:13.5: 1)
SS3 (Toluen/EtOAc/MeOH= 92:5:3)
These TLC chromatograms of ethyl acetate extract always show a very
broad active zone so that the antibacterial activity of this extract might be due to the
presence of more than one compound. The amount of 6.6 mg ethyl acetate extract was
dissolved in 200 mL MeOH, followed by shaking for 30 minutes. Afterwards, the
methanol phase was collected by pipetting. The residue which was not dissolved in
methanol was dried at room temperature and stored at -200C. Methanol phase was
dried in vacuum to give the methanol fraction.
Since this methanol fraction showed strong antibacterial activity against S.
aureus, the active MeOH fraction was analyzed by HPLC with DAD detection and a
Synergi-Polar RP (80A) column with a solvent gradient from 5%-100%
acetonitrile/water in 30 minutes. Due to the insufficient separation of the components
by HPLC, GC-MS was used for identifying the components.
2.11 Gas chromatography-mass spectrometry
Identification of fatty acids in the n-hexane extract from Lyngbya majuscula
biomass and the MeOH fraction obtained from ethyl acetate extract of Westiellopsis
sp.VN medium was done by gas chromatography-mass spectrometry with the help of
Dr. Martina Wurster, Department of Phamaceutical Biology, Institute of Pharmacy,
Ernst-Moritz-Arndt-University of Greifswald, Germany.
The n-hexane extract (1 mg) was hydrolyzed previously. For the hydrolysis
3-4 drops of n-hexane extract were treated with 3 KOH tablets and 5mL of ethanol in
a 250 mL flask. The mixture was heated to boiling for 10 min at reflux in a heating
mantle. After cooling, the mixture was diluted with 10 mL aqua dest. After this, the
pH of the chilled hydrolysate was adjusted with hydrochloric acid up to 2.0 and the
fatty acids were extracted 3 times with n-hexane (3x15 mL). The sample was reduced
in a rotary evaporator and then the rest was dried with a pinch of sodium sulfate, the
supernatant was transferred into an HPLC vial and dried over night at room

Chapter II Materials and methods
58
temperature to get the residue.
MeOH fraction (1 mg) was hydrolyzed previously as described for n-hexane
extract.
The derivatization (Liebeke et al., 2010) was performed by adding 40 µL of
methoxyamine (MeOX) [20mg ml-1 pyridine] to 1mg dried extract and heating for 3
min in microwave at 240 Watts (W). After this first heating phase, 80 µL of MSTFA
(N-methyl-N-trimethylsilyltrifluoroacetamide) was added and heated for further 3
minutes at 240 Watts in the microwave. The solution were then subjected to GC/MS
analysis under following conditions
Parameters for the Gas chromatograph
Carrier Gas: Helium
Carrier Flow: 1.0 mL/min
Injection Port Parameters: split, 25:1
Injection Port Temperature: 2300C
Purge flow to split vent: 20 mL/min after 2 min
Column: DB-5MS (30 m×0.25 mm×0.25 µm)
Column Oven Temperature: 700C initial (1 min),
Increasing to 760C by1.5 0C/min
Then to 3300C by 50C/min, keep in 10 min
Total: 65.8 min
Solvent Delay 6.2 min
Injection Volume: 2.0 µL
Injection Liner: Single taper liner
MS Transfer Line Temperature: 2500C
Parameter for the mass spectrometer
Mode: Electron Ionization (70 eV)
Tune: Auto tune with PFTBA (Masses 69, 219, 502)
Dwell time: 30 ms
Scan-Modus: 35-573 m/z, Scanrate 2.74 scans/sec
The detected compounds were identified by processing of the raw GC-MS
data with ChemStation G1701CA software and comparing with the NIST (National

Chapter II Materials and methods
59
Institute of Standards and Technology, Gaithersburg, USA) mass spectral database
2.0 d, and from retention times and mass spectra of standard compounds of the
library. Relative amounts of detected compounds were calculated based on the peak
areas of the total ion chromatograms (TIC).
2.12 Culture optimization of Westiellopsis sp.VN
2.12.1 The effects of nitrogen deficiency
To determine the effects of nitrogen deficiency on biomass production and
bioactive compound accumulation of Westiellopsis sp.VN the BG-11 (Rippka, 1979)
medium without NaNO3 was tested in large-scale culture. The standard BG11 medium
was used as control. The large-scale culture was performed in a 45 liter-glass
fermentor containg 35 L of liquid medium (see 2.5.3) at 28°C under continuous
illumination using cool-white fluorescent tubes of 8µmol/m2. The pH-value of the
large-scale culture was adjusted to 7.4 using CO2 supplementation. 1500 mL of
inoculum cultivated for 20 days in Fehrnbach flasks was added to each fermentor.
After 7 weeks, the cultures were harvested by centrifugation at 6500 rpm in a
refrigerated continuous-flow centrifuge. The biomasses were lyophilized (Lyophylizer
Alpha 1-4), weighted, and stored at -200C. 3 L of every cyanobacterial cultured
medium was concentrated to 300 mL by rotary evaporation in vacuum at 400C and
stored at -200C.
To investigate the effects of nitrogen deficiency on production of active
compounds, lyophilized biomass was extracted with n-hexane, ethyl acetate, and
methanol (see 2.6.1) and growth medium was extracted with ethyl acetate (see 2.6.2).
The prepared extracts were tested for antibiotic activity against Staphylococcus
aureus ATCC 6538 (see 2.7.1.1)
2.12.2 The effects of cultivation time (culture age)
The effects of cultivation time on the growth and production of active
compounds of Westiellopsis sp.VN strain were investigated in batch cultures. The
batch culture experimentw were carried out in 300 mL Erlenmeyer flasks containing
150 mL of liquid medium, BG-11 [Ripka, 1979] medium without NaNO3 under
continuous illumination provided by cool-white fluorescent tubes of 8 µmol/m2 at
room temperature (20 ±20C). 20 mL of inoculum cultivated in Fehrbach flasks for 20

Chapter II Materials and methods
60
days at the temperature and light conditions described above (according to 12.6 mg
lyophilized biomass) was added into each Erlenmeyer flask. The flasks were
incubated at the temperature and the light conditions described above and their places
were changed randomly everyday. Growth was monitored by measuring the dry
weight of biomass after 2, 3, 4, 5, 6, 7, and 8 weeks. At each sampling the contents of
three Erlenmeyer flasks were pooled in order to get enough cell material for the
analysis. The cells were harvested by centrifugation (3500 rpm/ 10 min/ 100C),
lyophilized (Lyophylizer Alpha 1-4) and weighted for determining growth curve.
To investigate the influence of cultivation time on production of active
compounds, the dried cells were extracted by methanol to get methanol extracts that
were tested for antibiotic activity against Staphylococcus aureus ATCC 6538 (see
2.7.1.1)

Chapter III Results
61
3 Results
3.1 Screening of antibacterial activity
3.1. 1 Extract preparation
12 cyanobacterial strains (see 2.1.1) were cultured in batch cultures (see
2.5.2). After harvesting, both biomass and culture media were used for extraction (see
2.6). The dry weight of extracts from biomass and culture media of the 12
cyanobacterial strains are shown in table 3-1.
Table 3-1: Dry weight of extracts from biomass (1g) and culture media (1L) of 12 cyanobacterial strains
Strain Strain number
n- Hexane extr. (mg)
MeOH extr. (mg)
H2O extr. (mg)
EtOAc extr. (mg)
Anabaena sp. TVN40 2.80 72.87 101.33 3.80 Nostoc spongiaforme TVN7 3.13 222.26 122.34 7.93 Nostoc coeruleum TVN14 2.60 79.47 112.00 2.53
Nostoc sp. TVN9 4.60 203.73 146.67 8.75 Calothrix elenkinii TVN202 5.80 279.20 152.00 7.20 Calothrix machica
var. crassa TVN201 5.13 144.27 75.33 6.68
Calothrix javanica TVN1 5.47 168.15 116.15 5.67 Calothrix sp. TVN20 5.54 150.91 120.42 8.42
Oscillatoria sp. TVN16 8.27 180.00 105.33 3.66 Scytonema ocellatum TVN10 10.55 183.54 102.70 4.20 Scytonema millei TVN12 6.42 152.15 118.9 1.60
Westiellopsis sp.VN TVN22 4.3 188.7 90.1 3.03 extr. = extract
In the majority of cases methanol extracts showed the highest yields while the
n-hexane extracts displayed the lowest yields. All the crude extracts were
subsequently used for antibacterial screening.
3.1.2 Screening of crude extracts
48 extracts of biomasses and culture media of 12 different cyanobacterial
strains were tested against two Gram-positive and two Gram negative bacteria in agar
plate diffusion test for antibacterial activity (see 2.7.1.1).
23 n-hexane, methanol, and ethyl acetate extracts exhibited activity against the
Gram positive bacterium Bacillus subtilis ATCC 6051. The ethyl acetate extract from
cultivation medium of Westiellopsis sp.VN (see fig.3-1) exhibited maximum
inhibition zone of 25 mm against this bacterium.

Chapter III Results
62
Figure 3-1: Antibacterial activity of cyanobacterial extracts against the Gram positive bacterium Bacillus subtilis ATCC 6501
(n=2; extract concentration 2 mg/6 mm paper disc, agar plate diffusion assay, inhibition zone including the diameter of paper disc).
22 n-hexane, methanol, and ethyl acetate extracts showed activity against
Staphylococcus aureus ATCC 6538 (see fig.3-2)
Figure 3-2: Antibacterial activity of cyanobacterial extracts against the Gram positive bacterium Staphylococcus aureus ATCC 6538
(n=2; extract concentration 2 mg/6 mm paper disc, agar plate diffusion assay, inhibition zone including the diameter of paper disc).
According to the diameter of inhibition zones (mm), the effect of the extracts
obtained from the same cyanobacterial strain showed no significant differences in

Chapter III Results
63
activity against Staphylococcus aureus and Bacillus subtilis. The methanol extract
obtained from biomass of Westiellopsis sp.VN showed the highest activity with
inhibition zone of 19 mm and the methanol extracts obtained from biomass of
Calothrix elenkinii, Scytonema mileii, Scytonema ocellatum, Calothrix javanica, and
Nostoc sp. exhibited moderate activity against Gram-positive bacteria. In addition, the
ethyl acetate extracts obtained from medium of the strains Westiellopsis sp.VN and
Anabaena sp. showed strong activity.
11 of 48 extracts showed activity against the Gram-negative bacterium
Escherichia coli. Of these active extracts, the ethyl acetate extract obtained from
cultivation medium of Westiellopsis sp.VN showed the highest activity with inhibition
zone of 25 mm, followed by the ethyl acetate extract obtained from cultivation
medium of Anabaena sp. with inhibition zone of 21mm. Interestingly, almost all
extracts which inhibited the growth of Escherichia coli were prepared from
cultivation medium (see fig.3-3). In case of Westiellopsis sp.VN the extracts of
biomass and cultivation medium exhibited strong activity.
Figure 3-3: Antibacterial activity of cyanobacterial extracts against the Gram negative bacterium Escherichia coli ATCC 11229
(n=2; extract concentration 2 mg/6 mm paper disc, agar plate diffusion assay, inhibition zone including the diameter of paper disc).
Activity against the gram negative bacterium Pseudomonas aeruginosa was
seldom found. 3 out of 48 extracts inhibited the growth of this bacterium but these
three extracts did not result in complete inhibition (see fig.3-4)

Chapter III Results
64
Figure 3-4: Antibacterial activity of cyanobacterial extracts against the Gram negative bacterium Pseudomonas aeruginosa ATCC 22853
(n=2; extract concentration 2 mg/6 mm paper disc, agar plate diffusion assay, inhibition zone including the diameter of paper disc).
In all, a total of 48 lipophilic and hydrophilic extracts obtained from 12
samples of cultured soil cyanobacteria have been screened for their antibacterial
activities. Of 48 extracts, 23 (47.92%; 3 n-hexane, 11 MeOH, and 9 EtOAc extracts)
showed activity against the Gram-positive bacterium B. subtilis, 22 (45.83%; 3 n-
hexane, 11 MeOH, and 8 EtOAc extracts) exhibited activity against the Gram-positive
bacterium S. aureus, 11 (22.92%; 1 n-hexane, 4 MeOH, and 6 EtOAc extracts)
inhibited the growth of the Gram-negative bacterium E. coli. Three MeOH extracts
showed effects on the growth of the Gram-negative bacterium P. aeruginosa but these
effects did not result in complete inhibition, and none of the water extracts was active
against the test bacteria. Of these active extracts, the methanol extract of Westiellopsis
sp.VN showed the highest activity against the Gram-positive bacteria and the ethyl
acetate extracts obtained from Westiellopsis sp.VN and Anabaena sp showed the
highest activity against Gram-negative bacterium E. coli. Interestingly, 12 of 12
investigated cyanobacteria are able to inhibit the growth of at least one of the test
organisms used Based on the results of the antibacterial screening, Westiellopsis
sp.VN, Anabaena sp., Calothix javaniva, Scytonema ocellatum and Nostoc sp. strains
were chosen for chemical investigation with emphasis on the isolation and structure
elucidation of antibacterial active secondary metabolites.

Chapter III Results
65
3.2 Chemical investigation and culture optimization of Westiellopsis
sp. VN
3.2.1 Chemical investigation of methanol extract obtained from biomass
Due to the highest antibacterial activity, the methanol extract resulting from
extraction of dry biomass was first chosen for chemical investigation with an
emphasis on the isolation and structure elucidation of active metabolites. This led to
the isolation and identification of the 6 following compounds ambiguine D isonitrile,
ambiguine B isonitrile, dechloro-ambiguine B isonitrile, fischerellin A, hydroxy-
eicosatetraenoic acid and methoxy-nonadecadienoic acid.
3.2.1.1 Fractionation of methanol extract by silica gel column chromatography
The methanol extract is usually a complex mixture of organic compounds from
non-polarity to polarity. Thus, the methanol extract of Westiellopsis sp. VN was
fractionated to remove a large portion of the unwanted material in a fairly low-
resolution step. 260 mg of methanol extract were subjected to silica gel column
eluting with a stepwise gradient of DCM/EtOAc/MeOH (see 2.8.1) to give 8 fractions
evaluated for antibacterial activity against S. aureus using agar diffusion method (see
table 3-2).
Table 3-2: Fractionation of methanol extract from Westiellopsis sp. VN biomass by silica gel chromatography and antibacterial activity of fractions to S. aureus
Yield
Fractions
Mobile phase (mg) %
recovered
Dose
(µg/disc)
Diameter of inhibition
zone (mm)1
FI DCM 58.6 22.54 500 21
FII DCM/EtOAc (95:5) 7.7 2.96 500 15
FIII DCM/EtOAc (90:10) 5.2 2.0 500 10
FIV DCM/EtOAc (50:50) 40.3 15.5 500 02
FV EtOAc 2.4 0.92 500 0
FVI EtOAc/MeOH (75:25) 4.7 1.81 500 0
FVII EtOAc/MeOH (50:50) 102.2 39.31 500 0
FVIII MeOH 34.9 13.42 500 0
Total 256.0 98.46
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect

Chapter III Results
66
Three contiguous fractions (FI, FII, and FIII) showed strong antibacterial activity,
and the highest activity was detected in fraction FI eluting with 100% DCM with
inhibition zone of 21 mm. Moderate activities were found in fractions FII and FIII with
inhibition zones of 15 mm and 10 mm, respectively. Because of strong antibacterial
activity of these three fractions, they were combined to the mixture which was further
purified.
3.2.1.2 Seperation of the combined fractions (FI, FII, and FIII) by sephadex LH-20
column
Sephadex LH-20 column chromatography involves separation based on
molecular size of compounds being analyzed. Thus, it was used for separating
components of the combined fractions FI, FII, and FIII because these three active
fractions were eluted with solvent systems not so different in polarity and therefore
may contain compounds with the same polar nature.
The combined fractions (68.5 mg) were subjected to a sephadex LH-20
column eluted with 90% MeOH in H2O followed by 100% MeOH, and finally with
50% aceton in H2O (see 2.8.1) to yield 4 fractions. The antibacterial activity of the
fractions against Staphylococcus aureus was tested in agar diffusion assay (see table
3-3).
Table 3-3: Fractionation of combined fractions FI, FII, and FIII from Westiellopsis sp. VN biomass by LH-20 chromatography and antibacterial activity of fractions to S. aureus
Yield
Fractions
No of sub
Fractions
pooled
Mobile phase (mg) %
recovered
Dose
(µg/disc)
Diameter of
inhibition
zone (mm)1
WF1 1-30 H2O/MeOH (10:90) 51.8 75.62 500 21
WF2 31-40 H2O/MeOH (10:90) 1.8 2.63 500 15
WF3 41-70 MeOH 8.6 12.55 500 10
WF4 70-90 H2O/ Aceton (50:50) 5.2 7.59 500 02
Total 67.4 98.39
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect
Fraction WF1 which eluted with 90%MeOH in H2O showed the highest
antibacterial activity with inhibition zone of 21 mm and was further purified.

Chapter III Results
67
3.2.1.3 Purification of fraction WF1 using reversed-phase HPLC
Final purification of fraction WF1 (50.0 mg) was achieved by semi-preparative
RP HPLC to give nine fractions. All fractions were evaluated for antibacterial activity
against S. aureus (see fig-5 and table 3-4).
WF1- 1
WF1- 2
WF1 -3
WF1- 4
WF1 - 5
WF1 - 6
WF1- 7
WF1 -8
WF1- 9
Figure 3-5: Semi-preparative RP HPLC chromatogram of WF1 (mobile phase table 2-2) 500 µg/50 µL/injection, detection at 238 nm, column Synergi POLAR-RP 80A (250×10 mm, 4 micron), flow rate
3 mL/min.
Table 3-4: Fractionation of WF1 from Westiellopsis sp. VN biomass by semi-preparative reversed-phase HPLC and antibacterial activity of fractions to S. aureus
Yield Fractions Retention
time (mg) % recovered
Dose
(µg/disc)
Diameter of inhibition
zone (mm)1
WF1-1 14.74 2.3 4.6 200 17.0
WF1-2 23.50 0.6 1.2 200 24.0
WF1-3 24.19 1.4 2.8 200 28.0
WF1-4 25.18 0.4 0.8 200 14.0
WF1-5 30.04 1.7 3.4 200 10.0
WF1-6 30.85 2.6 5.2 200 8.0
WF1-7 33.99 0.5 1.0 200 9.0
WF1-8 41.03 2.8 5.6 200 10.0
WF1-9 41.53-48.00 5.7 11.4 200 0.02
Total 18 36
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect

Chapter III Results
68
Based on the results of agar diffusion assay and yield of these nine fractions,
the four fractions WF1-3, WF1-5, WF1-6, and WF1-8 were used for structure elucidation.
3.2.1.4 Structure elucidation of isolated compounds of Westiellopsis sp.VN
3.2.1.4.1 Structure elucidation of fraction WF1-3 (compound 1)
Fraction WF1-3 was found to be the pure compound, ambiguine D isonitrile
(see fig.3-6).
NH
CH3
H3C
H3C
CH3CH3
H
OH NC
16
2
34
5
7
8
9
1011
1213
14
15
16
17
18
19
2021
22 23
24
25
26
27
28
Cl
O
Figure 3-6: Ambiguine D isonitrile, molecular formula C26H29ClN2O3
The high resolution ESI mass spectrum in positive mode of fraction WF1-3
indicated the presence of one compound in this fraction with the protonated molecular
ion peak [M+H]+ at m/z 453.1947 (see fig.3-7) corresponding to a molecular formula
of C26H29ClN2O3 (calculated 453.1939 for C26H30ClN2O3 ).
In addition, 1H NMR chemical shifts of compound1 are identical to those
reported for ambiguine D isonitrile isolated by Smitka et al., 1992 (see table 3-5,
appendix 1a,b). Thus, the identification of compound 1 was confirmed.
[ M + H ] +
[ M + H ] +
Figure 3-7: UV and ESI-MS of fraction WF1-3 (compound 1)

Chapter III Results
69
Table 3-5: 1H NMR data of compound 1 compared with literature data of ambiguine
D isonitrile
Position
Compound 1( in CDCl3)
δH
*Ambiguine D isonitrile in CDCl3
δH
5 7.12 dd 7.12 dd 6 7.31 m 7.31 m 7 7.32 m 7.32 m 13 4.09 ax 4.09 ax, dd 14 2.47 ax 2.47 ax, q 14 2.17 eq 2.17 eq, ddd 15 1.64 br ddd 17 1.39 s 1.39 s 18 1.35 s 1.35 s 19 1.68 s 1.68 s 20 5.96 dd 5.96 dd 21 5.53 E, d 5.53 E, d 21 5.37 Z, d 5.37 Z, d 25 3.11 d 3.11 d 26 3.55 d 3.55 d 27 1.72 s 1.72 s 28 1,73 s 1.73 s
3-OH 2.91 s 10-OH 4.26 4.26 d
*Smitka et al., 1992
3.2.1.4.2 Structure elucidation of fraction WF1-5
The positive ion ESI mass spectra of fraction WF1-5 indicated a mixture of
three compounds compatible with three peaks. These compounds revealed molecular
ion peaks as shown in figure 3-8.
peak1
peak2
peak3
Figure 3-8: UV and ESI-MS of compounds of fraction WF1- 5

Chapter III Results
70
The high resolution ESI mass spectrum in positive mode of peak1 showed the
presence of one compound with the protonated molecular ion peak [M+H]+ at m/z
423.2194 (see fig.3-9) corresponding to a molecular formula of C26H31ClN2O
(calculated 423.2198 for C26H32ClN2O) and this compound was also found in fraction
WF1-6 . Thus, the presence of this compound in this fraction was indicated.
peak1
[ M+ H] +
Figure 3-9: MS data of peak 1 of fraction WF1-5
Peak 2 was identified as the compound ambiguine C isonitrile (compound 2)
(see fig.3-10)
NH
CH3
H3C
H3C
CH3
CH3
H
OH
NC
16
2
34
5
7
8
9
1011
1213
14
15
16
17
18
19
20
21
22
23
24
25 26
27
28
Figure 3-10: Ambiguine C isonitrile, molecula formula C26H32N2O
The identification of ambiguine C isonitrile (dechloro-ambiguine B isonitrile)
with the molecular weight of 388 Dalton compatible with the molecular formula
C26H32N2O was deduced from the high resolution ESI mass spectrum in positive
mode which displayed a molecular ion peak [M+H]+ at m/z 389.2586 (calculated

Chapter III Results
71
389.2586 for C26H33N2O) and the [M+K]+ peak at m/z 427.2115 as shown in figure 3-
11
[ M + K] +
pe a k 2
[ M + H ] +
Figure 3-11: MS data of peak 2 of fraction WF1-5 (compound 2)
Peak 3 was identified as the compound fischerellin A (compound 3) (see figure
3-12).
N
N
O
O H
1 23
45
67
89
10
1112
1314
15
16
17
18
19
2021
22
23
24
25
26
Figure 3-12: Fischerellin A, molecular formula C26H36N2O2
The identification of fischerellin A (compound 3) was established by direct
comparison of our spectroscopic data including ESIMS in positive mode, and 1H
NMR with those reported in the literature (Hagmann and Jüttner, 1996). First,
compound 3 was assigned a molecular formula of C26H36N2O2 by HRESIMS (in
positive mode) data which displayed a prominent [M+H]+ peak at m/z 409.2846

Chapter III Results
72
(calculated 409.2850 for C26H37N2O2) and another [M+K]+ peak at m/z 447.2402 (see
fig 3.13). Next the identification of compound 3 was carried out by searching in DNP
using elemental composition indicating there were 5 hit compounds including only
fischerellin A isolated from cyanobacteria. Finally direct comparison of 1H NMR data
of compound 3 with those of reported in the literature was undertaken (see table 3-6,
appendix 2a, b)
peak3
[ M+ H] +
[ M+ K] +
Figure 3-13: MS data of peak 3 of fraction WF1-5 (compound 3)
Table 3- 6: 1H NMR data of compound 3 compared with literature data* of
fischerellin A
Position Multiplicity Proton shift of
compound 3 in CDCl3
*Proton shift of fischerellin
A in CDCl3
6 CH 3.5-3.6 3.56
8 CH 3.8-3.9 3.85
3.83
12 CH 6.2-6.3 6.24
13 CH 5.45-5.5 5.49
26 CH3 2.9-3.0 2.92
2.93
*Hagmann and Jüttner, 1996

Chapter III Results
73
3.2.1.4.3 Structure elucidation of fraction WF1-6 (compound 4)
WF1-6 was found to be the pure compound, ambiguine B isonitrile (see fig. 3-
14)
NH
ClCH3
H3C
H3C
CH3
CH3
H
OH
NC
16
2
34
5
7
8
9
1011
121314
15
16
17
18
19
20
21
22
23
24
25 26
27
28
Figure 3-14: Ambiguine B isonitrile, molecular formula C26H31ClN2O
The high resolution ESI mass spectrum in positive mode of fraction WF1-6
indicated the presence of one compound in this fraction with the protonated molecular
ion peak [M+H]+ at m/z 423.2195 (see fig.3-15) corresponding to a molecular formula
of C26H31ClN2O (calculated 423.2198 for C26H32ClN2O).
In addition, the 1H NMR signals of compound 4 were fully consistent with
literature values for the known metabolite ambiguine B isonitrile reported earlier
(Smitka et al., 1992) (table 3-7 and appendix 3a, b)
[M+H]+
Figure 3-15: UV and ESI-MS of fraction WF1-6 (compound 4)

Chapter III Results
74
Table 3-7: Comparison of 1H NMR data of compound 4 with reported data*
Position
Compound 4 ( in CDCl3)
δH
*Ambiguine B isonitrile in CDCl3
δH
1 8.15 br s 8.18 br s 5 7.06 dd 7.07 dd 6 7.15 m 7.17 m 7 7.13 m 7.15 m 11 - 4.68 eq, s 13 - 4.41 ax, dd 14 2.53 ax, q 2.53 ax, q 14 2.30 eq, ddd 2.30 eq, ddd 15 2.41 ddd 2.41 ddd 17 1.23 s 1.23 s 18 1.54 s 1.54 s 19 1.53 s 1.53 s 20 6.04 dd 6.04 dd 21 5.31 E, d 5.31 E, d 21 5.26 Z, d 5.26 Z, d 25 6.33 dd 6.33 dd 26 5.28 E, d
5.36 Z, d 5.28 E, d 5.36 Z, d
27 1.62 s 1.62 s 28 1.64 s 1.64 s
10-OH - 1.67 s * Smitka et al., 1992
3.2.1.4.4 Identification of compounds in fraction WF1-8
The high resolution ESI mass spectrum in positive mode of fraction WF1-8
displayed a mixture of three peaks (see fig.3-16). Peak 1 with m/z 301.1413 is typical
for softener. Its presence was also shown in the 1H NMR spectrum (appendix 4a, b).
peak1
peak2
peak3
Figure 3-16: ESI-MS of compounds of fraction WF1- 8

Chapter III Results
75
The high resolution ESI mass spectrum in positive mode of peak 2 (see fig.3-
17) showed a protonated molecular ion peak at m/z 335.2555 [M+H]+ and other two
peaks at m/z 351.2498 [M+O]+ and m/z 367.2242 [M+O2]+, leading to the molecular
weight of 334 Dalton. Searching in DNP using molecular weight and 1H NMR
spectrum of this fraction which showed typical signals for unsaturated fatty acids
(appendix 4a, b) led to the methoxy-eicosatetraenoic acid (compound 5) as the most
probable compound with molecular formula C21H34O3 (calculated 335.2581 for
C21H35O3).
[ M + O] +
[ M+ H] +
Figure 3-17: ESI-MS of peak 2 of fraction WF1- 8 (compound 5)
The high resolution ESI mass spectrum in positive mode of peak 3 (see
fig.3-18) delivered signals at m/z 311.2596 [M+H]+ and 333.2398 [M+Na]+ as shown
in figure 3.18, leading to the molecular weight of 310 Dalton. Searching in DNP using
molecular weight and 1H NMR spectrum of this fraction which showed typical signals
for unsaturated fatty acids (shown in appendix 4a,b) led to the hydroxy-
nonadecadienoic acid-derivative (compound 6) as the most probable compound with
molecular formula C19H34O3 (calculated 311.2581 for C19 H35O3).

Chapter III Results
76
[ M+ Na] +
[ M+ H] +
Figure 3-18: ESI-MS of peak 3 of fraction WF1- 8 (compound 6)
3.2.2 Chemical investigation of the active ethyl acetate extract resulting from
cultivation medium of Westiellopsis sp. VN
In antimicrobial screening the ethyl acetate extract from cultivation medium
of this cyanobacterial strain exhibited a strong antibacterial activity against Gram-
positive bacteria B. subtilis, S. aureus and Gram-negative bacteria E. coli, P.
aeruginosa as well as yeast Candida maltosa SBUG 700 (IZ of 13.5 mm). This
extract was therefore highly appropriate for investigation of antimicrobial compounds.
Bioautographic TLC assay was successful used to locate the active
principles of ethyl acetate extract from cultivation medium of Westiellopsis sp.VN on
TLC chromatograms with three mobile phases SS1, SS2, and SS3, separately.
The TLC chromatograms of ethyl acetate extract always show a very broad
active zone (see fig.3-19), so that the antibacterial activity of the extract might be due
to the presence of more than one compound.

Chapter III Results
77
SS1 SS2 SS3
Figure 3-19: Bioautographic assay of EtOAc extract from culture medium of Westiellopsis sp.VN
against S.aureu; SS1 (n-hexane/EtOAc/MeOH= 75:25:5), SS2 (EtOAc/MeOH/H2O= 100:13.5: 1), SS3 (Toluen/EtOAc/MeOH= 92:5:3)
The ethyl acetate extract of cultivation medium was further separated by
shaking with methanol.The methanol fraction obtained from ethyl acetate extract (see
2.10) showed strong antibacterial activity against S. aureus with inhibition zone of 28
mm (C=2.0 mg/disc) in agar diffusion assay. Thus, this active fraction was analyzed
by HPLC with a solvent gradient from 5%-100% acetonitrile/water (see fig.3-20).
Figure 3-20: Analytical HPLC of MeOH fraction of EtOAc extract of cultivation medium of Westiellopsis sp. VN with mobile phase 5%-100% acetonitrile/water for 30minutes, Synergi POLAR-
RP 80A column (250×4.6mm, 4 micron), flow rate 1mL/min, detection at 210 nm
Due to the insufficient separation of the components by HPLC, GC-MS was
used for identifying the volatile components in this methanol fraction. This fraction
was solved in n-hexane and methanol respectively and analyzed after hydrolysis and
derivatization (see table 3-8 and 3-9, appendix 5 and 6).

Chapter III Results
78
Table 3-8: Compounds of MeOH fraction analyzed as methyl ester in n-hexane after hydrolysis /derivatization
ret.
time (min)
trivial name main mass spectral
fragment (m/z)
percent
(%)
match
(%)
21.172 L-Proline, 5-oxo-1-
(trimethylsilyl)-, trimethylsilyl
ester (C11 H23NO3Si2)
230, 156, 73, 45 7.330 91.9
32.465 Palmitic acid (C16:0) 313, 269,201, 117, 73,
43
4.169 89.0
35.531 Stearic acids (C18: 0) 339, 222, 117, 73, 55 22.748 84.8
35.532 11-cis- Octadecanoic acid (C18:1) 399, 264, 117, 73, 55,
41
1.124 81.6
51.384 ß-Sitosterol (C29H50O) 486, 396, 357, 255,
215, 161, 129
1.210 77.5
ret. time = retention time; m/z = the mass of an ion divided by the electrical charge of the ion
Table 3-9: Compounds of MeOH fraction analyzed as methyl ester in MeOH after hydrolysis/derivatization
ret.
time [min]
trivial name main mass spectral
fragment (m/z)
percent
(%)
match
(%)
13.796 Carbamic acid (CH3NO2) 278, 205, 147, 73, 453 0.159 84.1
21.165 L-Proline, 5-oxo-1-
(trimethylsilyl)-, trimethylsilyl
ester (C11H23NO3Si2)
230, 156, 73, 45 2.049 91.1
24.373 Lauric acid (C12H24O2) 257, 201, 117, 73, 55 1.143 86.2
24.881 Naphthalene (C15H18) 183, 83, 39 0.429 85.5
28. 609 Myristic acid (C14:0) 458, 368, 392, 247,
129, 73
1.298 88.1
32.055 Palmitic acid, isopropyl ester
(C16:0)
298, 256, 213, 157,
129, 102, 60, 43
0.734 71.1
32.465 Palmitic acid (C16:0) 313, 269,201, 117, 73,
43
2.456 88.3
36.015 Octadecanoic acid (C18:0) 341, 201, 117, 73, 43 1.345 90.9
ret. time = retention time; m/z = the mass of an ion divided by the electrical charge of the ion
GC-MS analysis of MeOH fraction obtained from ethyl acetate extract of the
cultivation medium, revealed the presence of different saturated fatty acids and the

Chapter III Results
79
unsaturated 11-cis- octadenoic acid. Furthermore naphthalene, carbamic acid and 5-
oxoproline were found. However, the GC-MS technique is most suitable for
thermostable and volatile substances, but not for non-volatile ones and chromatogram
obtained from analytical HPLC of this methanol fraction revealed the presence of
other compounds. Thus, further separation of the mixture of other substances in this
methanol fraction is necessary to verify whether only compounds identified so far
(see table 3.8 and 3.9) were responsible for very strong antimicrobial activity of this
methanol fraction.
3.2.3 Culture optimization of Westiellopsis sp.VN
3.2.3.1 Nitrogen deficiency
Cultivation of Westiellopsis sp. VN in BG11 without nitrate and in normal
BG11 medium containing 1.5 g/L NaNO3 (see fig. 3-21) showed that the amounts of
dry biomass and the yields of methanol extracts did not differ significantly at different
nitrogen concentrations. The yields of the lipophilic n-hexane and EtOAc extracts in
the nitrogen-free medium were higher than those in nitrogen-containing medium.
Figure 3-21: Fermenters for cultivation of Westiellopsis sp.VN containing standard BG11 medium and BG-11 medium without NaNO3, illuminated continuously with 8µmol/m2 at 28°C, pH 7.4, CO2
supplementation
Based on diameter of inhibition zone (IZ), the concentration of antibacterial
compounds in methanol extracts of biomass and ethyl acetate extracts of growth

Chapter III Results
80
medium did not change markedly while antibacterial compound concentration of n-
hexane and ethyl acetate extracts of biomass changed significantly (see table 3-10, fig.
3-22).
Table 3-10: Dry biomass and antibacterial activity against S.aureus of extracts in BG-11 medium
BG11 without NaNO3
(BG-110)
BG11 with NaNO3
(BG-11)
Lyophilized biomass 14.750g 14.884 g
Yield of methanol extract from biomass 1153.6 mg 1192.3 mg
Yield of EtOAc extract from biomass 157.7 mg 91.8 mg
Yield of EtOAc extract from medium 98.7 mg 70.0 mg
Yield of n-hexane extract from biomass 132.1 mg 51.1 mg
IZ of n- hexane extract from biomass 18.0 mm 9.0 mm
IZ of EtOAc extract from biomass 18.0 mm 12.5 mm
IZ of MeOH extract from biomass 17.0 mm 19.0 mm
IZ of EtOAc extract from medium 29.0 mm 27.0 mm
IZ {Diameter of I nhibition zone including diameter of paper disc (6 mm)}
hex .
EtOAc.
MeOH
hex .
EtOAc
MeOH
BG110 BG11
BG110 BG11
EtOAc. EtOAc.
Biomass Grow th m edium
Figure 3-22: Agar diffusion test of extracts prepared from biomass and cultivation medium of Westiellopsis sp.VN grown in BG-11 media S.aureus, C=2mg/disc

Chapter III Results
81
3.2.3.2 The effect of incubation time on biomass production and antibacterial
production
The biomass yield and dry weight respectively increased continuously to the
end of the 7-to 8- week growth period. Extracts prepared from biomass harvested after
4 weeks of cultivation showed an increased diameter of inhibition zone in agar
diffusion assay against S. aureus. Synthesis of antibiotic substances seems to be
constant over the next 2 weeks and increases slightly during the last two weeks of
cultivation time (see fig.3-23a and 3-23b).
0
5
10
15
20
25
30
35
40
45
50
0 1 2 3 4 5 6 7 8 9
I ncuba t ion t im e ( weeks)
Dry w
eight (mg/100ml culture)
0
5
10
15
20
25
Dry weight
Diameter of Inhibition zone
Figure 3-23a: The effect of incubation time on dry weight and antibiotic production of Westiellopsis sp.VN
Figure 3-23b: Agar diffusion test of MeOH extracts of Westiellopsis sp.VN biomass Methanol extracts prepared from biomass cultivated for 2, 3, 4, 5, 6, 7, and 8 weeks, respectively. Test organisms S.aureus, C=2mg/disc
Dia
mete
r of
Inhib
itio
n z
one (m
m)

Chapter III Results
82
3.3 Chemical investigation of Calothrix javanica
The lyophilized biomass obtained from batch culture (see 2.5.2) was
extracted with n-hexane, methanol, and water, respectively to give three extracts. The
methanol extract exhibited the strongest activity against B. subtilic and S.aureus and
was therefore selected for chemical investigation. The investigation of this active
methanol extract led to isolation of a new cyclic peptide named daklakapeptin
according to the locality of the strain.
3.3.1 Fractionation of methanol extract from biomass by RPC18 chromatography
210 mg of MeOH extract was chromatographed on a reverse-phase C18
column eluting with 5 solvent systems starting with MeOH/EtOH/H2O (45:45:10),
followed by MeOH/H2O (9:1), MeOH, and washing with MeOH/C3H6O (1:1) and
CH2Cl2, respectively to yield 10 fractions (CJFI to CJFX) (see 2.8.2). All obtained
fractions were evaluated for their antibacterial activities against S. aureus in agar
diffusion assay. The result of this assay revealed that fraction CJFII eluting with
MeOH/EtOH/H2O (45:45:10) possesses the strongest activity (see table 3-11),
therefore it was further purified.
Table 3-11: Fractionation of methanol extract from Calothrix javanica biomass by RP C18 chromatography and antibacterial activity of fractions to S. aureus
Yield
Fractions
No of
sub
fractions
pooled
Mobile phase (mg) %
recovered
Dose
(µg/disc)
Diameter
of IZ
(mm)1
CJFI 1-8 MeOH/EtOH/H2O (45: 45:10) 33.8 16.10 500 02
CJFII 9-24 MeOH/EtOH/H2O (45: 45:10) 89.9 42.81 500 9.0
CJFIII 25-30 MeOH/EtOH/H2O (45: 45:10) 1.8 0.86 500 7.0
CJFIV 31-34 MeOH/EtOH/H2O (45: 45:10) 1.2 0.57 500 0
CJFV 35-40 MeOH/EtOH/H2O (45: 45:10) 1.4 0.67 500 0
CJFVI 41-50 MeOH/EtOH/H2O (45: 45:10) 8.4 4.00 500 7.0
CJFVII 51-100 MeOH/ H2O (90:10) 17.8 8.48 500 7.5
CJFVIII 101-150 MeOH 14.5 6.90 500 0
CJFIX 151-200 MeOH/CH3COCH3 (50:50) 14.2 6.76 500 0
CJFX 201-250 DCM 22.8 10.86 500 0
Total 205.8 98.01
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect

Chapter III Results
83
3.3.2 Purification of fraction CJFII by semi-preparative reversed-phase HPLC
First, the active fraction CJFII was analyzed by HPLC with a solvent gradient
of acetonitrile/water (table 3-12, figure 3-24).
Table 3-12: Step gradient used in HPLC analysis of CJFII by analytical HPLC
Time (min) 0.50 25.5 26.50 30.50 32.00 Solvent A (%) 95 5 0 0.0 95 Solvent B (%) 5 95 100 100 5
Solvent A: H2O and solvent B: CH3CN; Flow rate: 1.0 mL/min; HPLC column: Synergi polar-
RP80A/250×4.6mm, 4 micron
Figure 3-24: Analytical HPLC chromatogram of fraction CJFII with mobile phase of acetonitrile/water (table 3-12) and 2mg/mL /injection, Synergi POLAR-RP 80A column (250×4.6mm, 4 micron), flow rate 1mL/min, detection at 220, 246 nm, respectively.
Chromatogram obtained from analytical HPLC revealed that purification of
fraction CJFII by reversed-phase HPLC was possible.
To optimize separation process, the mobile phase was changed and the mobile
phase of MeOH/ water acidified with 0.05% TFA was tested. With this mobile phase
a better separation was achieved (see table 2-3) used for a successful separation of
fraction CJFII by semi-preparative HPLC. Altogether, 86 mg of fraction CJFII were
purified. 7 peaks CJFII-1 to CJFII-7 were collected and controlled at the wave length of
220 nm to afford 7 seven fractions (see table 3-13).

Chapter III Results
84
Table 3-13: Separation of CJFII from Calothrix javanica biomass by semi-preparative reversed-phase HPLC and activity of the fractions to S. aureus
Yield Fractions Retention
time (mg) % recovered
Dose
(µg/disc)
Diameter of inhibition
zone (mm)1
CJFII -1 3.66 1.3 1.51 100 6.5
CJFII -2 4.54 43.4 50.47 200 02
CJFII -3 11.38 0.7 0.81 100 0
CJFII -4 12.93 3.8 4.42 200 12.5
CJFII -5 21.28 3.0 3.49 100 7.0
CJFII -6 26.63 4.4 5.12 100 15.0
CJFII -7 30.44 1.6 1.86 100 0
Total 58.2 67.68
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect
Seven fractions were analyzed by analytical HPLC using a step gradient of
MeOH/ water + 0.05% TFA as mobile phase described in table 3-14. In analytical
HPLC fraction CJFII-4 was the most pure one (see fig.3-25) and therefore used for
structural elucidation.
Table 3-14: Step gradient used in HPLC analysis of seven fractions by analytical HPLC
Time (min) 0.5 16.5 24.5 30.5 31.0 Solvent A (%) 50 15 0 0 50 Solvent B (%) 50 85 100 100 50
Solvent A: H2O+0.05%TFA and solvent B: MeOH; Flow rate: 1.0 mL/min; HPLC column: Synergi
polar-RP80A/250×4.6mm, 4 micron
Figure 3-25: Analytical HPLC chromatogram of fraction CJFII-4 (mobile phase see table 3-14), 2mg/mL /injection, detection at 220 nm, column Synergi POLAR-RP 80A (250×4.6 mm, 4 micron), flow rate 1mL/min.

Chapter III Results
85
3.3.3 Structure elucidation of fraction CJFII-4 (compound 7)
Fraction CJFII-4 was found to be a new cyclic peptide named daklakapeptin.
Figure 3-26: Daklakapeptin (compound 7)
The structure of the peptide was elucidated using a combination of high-
resolution ESI MS and 2D NMR techniques. Initially spin systems in the
homonuclear 2D 1H TOCSY spectrum were identified starting from the backbone
amide protons in the region 8.2 to 7.7 ppm. All protons of ten residues were found of
which five could be specifically identified from their characteristic chemical shifts,
namely Tyr, Gln, Leu, Ile and Thr (see table 3-15, systems 3, 4, 5, 7 and 10). The
shifts of four others (1, 2, 6 and 9) indicated the presence of unusual spin systems.
Careful integration of the 1D spectrum at 7.8 ppm indicated the α- and β-protons of a
Thr residue (system 8) overlap at 4.21 ppm. The presence of Tyr and Gln (3 and 4)
was confirmed from the identification of their aromatic and terminal CONH2 group,
respectively, in the region 7.5 to 6.5 ppm. Two further spin systems lacking amide
protons were then identified from correlations in the Hα region of the spectrum, 5.0 to
4.0 ppm. These, from the characteristic shifts, corresponded to Ile and Pro residues
(see table 3-15, systems 11 and 12). Overlap in the amide region of the spectrum that
caused some ambiguities in system identification was clarified by comparison with
the spin system patterns in the Hα region and the high-field region of the spectrum.
Comparison of these data with the cross peaks in the 2D COSY spectrum confirmed
the intra-residue assignments and established the nature of the unusual spin systems
(see table 3-15).
Sequence specific assignments were determined from the cross-peaks in the
2D 1H NOESY spectrum based on short observable distances between HN, Hα and

Chapter III Results
86
Hß of amino acid i and HN of amino acid i+1. The data, table 3-16, afforded two
possible combinations for the major sequence fragment of 10-2-12-3-8-9-7-1-6-4/5 or
2-10-12-3-8-9-7-1-6-4/5 together with a minor fragment 4-11. This same spectrum
indicated that the substituted Ile residue (system 11) was in fact an N-methyl
substituted unit. As in previous work heteronuclear 2D correlations were then
recorded with the intention of providing further sequence information through the
observation of correlations in the HMBC spectrum of HN with the amide carbonyl
carbons. Unfortunately most of carbonyl carbons were restricted to a narrow band in
the 13C spectrum that prevented an unambiguous assignment. Thus only the sequence
9-7 was confirmed. However the combination of the HMQC and HMBC data did
allow a complete assignment of the 13C shifts of the side-chains of the individual units
(see table 3-15) that confirmed the nature of these residues.
The structure of the molecule was finally established using high-resolution
ESI mass spectrometric data. The protonated molecular ion in the HR-ESI-MS of
[M+H]+ at 1432.8289 corresponded with a molecular composition of C68H114O20N13
which agreed with the molecular formula C68H113O20N13 and mass calculated
independently from the structure of the individual residues deduced from the NMR
data (see table 3-17) and indicated the molecule was a cyclic peptide. A detailed
analysis was then made of the fragmentation pattern in the high-resolution ESI MS.
This indicated the cyclic peptide initially underwent cleavage at the amide bond
directly prior to the proline residue. Subsequent fragmentation of this linear molecular
ion then afforded an unambiguous sequence that is independent, but compatible with
one of the possible sequences deduced from the NMR data, (b) in the footnote of table
3-16. The comparison of the sequence from the fragmentation in the HR-ESI-MS and
the NMR data is shown in table 3-18 and the corresponding complete structure of the
molecule is shown in the figure 3.26.
Table 3-15: NMR data of CJFII-4 Residue C/H No. δC δH
1 NH 8.14 CO Cα 82.0 4.57 Cβ 79.7 3.74 Cγ 32.8 1.75 Cδ 20.3, 19.9 1.09, 0.92
2-Complex NH 8.12 CO 174.3 Cα 64.8 4.65 Cβ 73.1 4.27 Cγ 64.8 3.60

Chapter III Results
87
3-Tyr NH 8.04 CO Cα 57.3 4.64 Cβ 38.2 3.17, 2.91 Arom C1:130.3, C2,6: 132.2,
C3,5:117.6, C4: 157.0 H2/6: 7.13, H3/5: 6.86
4-Gln NH 8.03 CO Cα 51.6 4.85 Cβ 28.3 2.08, 1.93 Cγ 32.8 2.32 CONH2 179.5 7.28, 6.59
5-Leu NH 8.03 CO Cα 55.1 4.37 Cβ 42.2 1.65 Cγ 26.3 1.60 Cδ 23.7, 21.9 0.95, 0.91
6-Complex NH 7.99 CO Cα 53.3 4.60 Cβ 35.3 2.18, 1.95 Cγ 60.0 3.70, 3.59
7-Ile NH 7.85 CO Cα 61.4 4.26 Cβ 38.3 1.93 Cγ 27.0, 16.5 1.57, 1.25, 0.98 Me Cδ 11.6 0.91
8-Thr NH 7.79 CO Cα 62.8 4.21 Cβ 69.1 4.21 Cγ 20.3 1.09 9§ CO -1 175.6 CH2-2 42.1 2.56 CH(NH-)-3 49.9 4.18, 7.78 NH CH2-4 35.1 1.57 CH2-5 27.6 1.38 CH-6 33.2 1.31 (CH3)2-7 24.0, 14.7 1.31, 0.88
10-Thr NH 7.77 CO Cα 59.0 4.66 Cβ 68.8 4.28 Cγ 20.8 1.24
11-IleNMe N-CH3 32.9 3.19 CO Cα 64.6 4.67 Cβ 34.2 2.11 Cγ 26.7, 16.5 1.40, 1.05; 0.97 Me Cδ 11.2 0.90
12-Pro CO 175.5 Cα 63.4 4.39 Cβ 30.9 2.13, 1.70 Cγ 26.5 2.00, 1.96 Cδ 50.1 3.82, 3.68
Footnote § 9 is (CH3)2.CH.CH2.CH2.CH(NH-).CH2 CO-

Chapter III Results
88
Table 3-16: Sequence information deduced from the NOE’s found in the 2D NOESY spectrum of CJFII-4
α-N (i, i+1) N-N (i, i +1) Others α-N(i,i+2)
12 (Pro)-3 12α-8NH? 3-8 3-8 3ß-8NH(x2)
3H2/6 – 8NH
8-9 9(CH2)-7 9(CHß)-7NH
7-1 7-1 7ß-1NH, 7γ-1NH 7α-6NH? 1-6
6-4or 5 4-11Nme
2-10 or 10-2 2α and 10α overlap
2α /10α-12α(x2), 2α/10α-12ß(x2) (or
reverse)
4γ-CONH2γ Summary: Possible sequence: (a) 2-10-12-3-8-9-7-1-6-4/5 or (b) 10-2-12-3-8-9-7-1-6-4/5, 4-11
Table 3-17: Calculation of the molecular mass from the structure of the residues deduced from the NMR data.
Residue Molecular Formula Mass
1 (CH3)2 CHCH(OH)CH(NH)CO
C6 H11 NO2 129
2 HOCH2CHOHCH(NH)CO
C4 H7 NO3 117
3-Tyr C9 H9 NO2 163.2 4-Gln C5 H8 N2 O2 128.1 5-Leu C6 H11 NO 113.2 6
HOCH2CH2CH(NH)CO C4 H7 NO2 101
7-Ile C6 H11 NO 113.2 8-Thr C4 H7 NO2 71.1 9
(CH3)2.CH.CH2.CH2.CH(NH-).CH2CO- C8 H15 NO 141
10-Thr C4 H7 NO2 101.1 11-IleNMe C7 H13 NO 127.2 12-Pro C5 H7 NO 97.1
Total C68H113N13O20 1431

Chapter III Results
89
Table 3-18: Comparison of the sequence from the high-resolution ESI-MS data with that from the NMR data.
Sequential loss of exact fragment masses in the HR-ESI-MS
261.1234 C14H17O3N
2 [M+H]+
101.0477 141.1154 113.0841 129.0788 101.0479 113.0840 128.0586 127.0988 50.5237 x2 58.5212 x2
Molecular formula of fragments sequentially lost in the HR-ESI-MS
C5H7NO + C9H9NO2
C4H7NO2 C8H15NO C6H11NO C6H11NO2 C4H7NO2 C6H11NO C5H8N2O2 C7H13NO C4H7NO2 C4H7NO3
Sequence from the NMR analysis
Pro - Tyr Thr (Leu+2CH2) a
Leu Leu+O b
Thr Leu Gln Ile-NMe Thr Thr+O c
Footnote: The structures of the complex residues are as follows: a) (CH3)2CH.CH2CH2CH(NH-)CH2CO-, b) (CH3)2CHCH(OH)CH(NH-)CO-, c) HOCH2CHOHCH(NH-)CO-.

Chapter III Results
90
3.4 Chemical investigation of Scytonema ocellatum
The investigation of the active methanol extract obtained from lyophilized
biomass from batch culture of this strain led to isolation of a cyclic peptide which was
the same as that isolated from Calothrix javanica.
3.4.1 Fractionation of methanol extract obtained from biomass by RP C18
column chromatography
In the first purification step, 230 mg of the methanol extract were
fractionated on RP C18 column with three solvent systems (see 2.8.3) to get 8
fractions (SOFI to SOFVIII) which were evaluated for antibacterial activity against S.
aureus using agar diffusion method (see table 3-19).
Table 3-19: Fractionation of methanol extract from Scytonema ocellatum biomass by RP-18 chromatography and antibacterial activity of fractions to S. aureus
Yield
Fractions
No of sub
fractions
pooled
Mobile phase (mg) %
recovered
Dose
(mg/disc)
Diameter
of IZ
(mm)1
SOFI 1-2 MeOH/EtOH/H2O
(45:45:10)
66.6 28.96 1.0 02
SOFII 3-10 MeOH/EtOH/H2O
(45:45:10)
76.3 32.74 1.0 10.0
SOFIII 11-12 MeOH/EtOH/H2O
(45:45:10)
1.7 0.74 1.0 0
SOFIV 13-20 MeOH/EtOH/H2O
(45:45:10)
9.5 4.13 1.0 0
SOFV 21-23 MeOH/EtOH/H2O
(45:45:10)
8.4 3.65 1.0 0
SOFVI 24-40 MeOH/EtOH/H2O
(45:45:10)
19.3 8.39 1.0 0
SOFVII 41-80 MeOH 43.3 18.83 1.0 0
SOFVIII 81-120 MeOH/CH3COCH3
(10:10)
2.5 1.09 1.0 0
Total 226.6 98.53
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect
Because only fraction SOFII showed strong antibacterial activity, this fraction
was further separated.

Chapter III Results
91
3.4.2 Separation of fraction SOFII using silica gel column
To remove more unwanted substances still remaining in the active fraction
SOFII, silica gel column chromatography was employed.
74.3 mg of fraction SOFII were subjected to silica gel column eluting with a
step gradient of n-hexane/EtOAc/MeOH (see 2.8.3) to yield 10 fractions. All these
fractions were tested for their antibacterial activity against S. aureus in agar diffusion
assay (see table 3-20)
Table 3-20: Separation of SOFII from Scytonema ocellatum biomass by silical gel chromatography and antibacterial activity of the fraction to S. aureus
Yield
Fractions
No of sub
fractions
pooled
Mobile phase (mg) %
recovered
Dose
(µg/disc)
Diameter
of IZ
(mm)1
SOFII-1 1-4 n-hexane/EtOAc
(25:75)
5.9 7.94 500 02
SOFII-2 5-20 n-hexane/EtOAc
(25:75)
1.1 1.48 500 0
SOFII-3 21-36 EtOAc 1.1 1.48 500 0
SOFII-4 37-43 EtOAc/MeOH (50:50) 1.1 1.48 500 0
SOFII-5 44-45 EtOAc/MeOH (50:50) 17.8 23.96 500 9.0
SOFII-6 46-51 EtOAc/MeOH (50:50) 11.7 15.75 500 8.0
SOFII-7 52-63 MeOH 5.8 7.81 500 0
SOFII-8 64-67 MeOH 10.9 14.68 500 0
SOFII-9 68-70 MeOH 3.8 5.11 500 0
SOFII-10 71-72 MeOH 2.4 3.23 500 0
Total 61.6 82.92
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect
The fractions SOFII-5 and SOFII-6 eluting with 50% EtOAc/MeOH exhibited
approximately the same antibacterial activity. Thus, these two fractions were pooled
and further purified.
3.4.3 Purification of the pooled fractions using reversed-phase RP HPLC
Final purification of the pooled fractions SOFII-5 and SOFII-6 (26.0 mg) was
achieved by reversed-phase HPLC (see 2.8.3) to yield three fractions. All these
fractions were evaluated for antibacterial activity against S. aureus (see fig.3-27 and

Chapter III Results
92
table 3-21).
FSO1
FSO3
FSO2
Figure 3-27: Semi-preparative RP HPLC chromatogram of the pooled fractions SOFII-5 and SOFII-6 with mobile phase (table 2-4) and 500µg/50 µL /injection, detection at 220 nm, column Synergi POLAR-RP 80A (250×10mm, 4 micron), flow rate 3mL/min.
Table 3-21: Separation of SOFII-5 and SOFII-6 from Scytonema ocellatum biomass by semi-preparative reversed-phase HPLC and antibacterial activity of the fractions to S. aureus
Yield Fractions Retention
time (mg) % recovered
Dose (µg/disc) Diameter of inhibition
zone (mm)1
FSO1 3.08 1.0 3.85 200 02
FSO2 4.29 13.4 51.54 200 0
FSO3 14.32 2.4 9.23 200 12.0
Total 16.8 64.62
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect
The active fraction FSO3 was analyzed by analytical HPLC (see fig.3-28)
with a step gradient as described in table 3- 22 and then used for structure elucidation.

Chapter III Results
93
Table 3-22: Step gradient used in HPLC analysis of FSO3 by analytical HPLC
Time (min) 0.50 18.50 21.50 24.50 25.00 Solvent A (%) 95 5 0 0.0 95 Solvent B (%) 5 95 100 100 5
Solvent A: H2O and solvent B: CH3CN; Flow rate: 1.0 mL/min; HPLC column: Synergi polar-
RP80A/250×4.6mm, 4 micron
Figure 3-28: Analytical HPLC chromatogram of fraction FSO3 (mobile phase see table 3-22), 2mg/mL /injection, detection at 220 nm, column Synergi POLAR-RP 80A (250×4.6 mm, 4 micron), flow rate 1mL/min.
3.4.4 Structure elucidation of fraction FSO3
Fraction FSO3 was found to be the same new cyclic peptide also found in the
biomass of Calothrix javanica (see 3.3.3).

Chapter III Results
94
3.5 Chemical investigation of Anabaena sp.
The investigation of the ethyl acetate extract resulting from the cultivation
medium of this strain led to isolation of fluourensadiol.
Because the crude extracts obtained from Anabaena sp. strain revealed a
strong and wide range of antimicrobial activity in our antibacterial screening (see
3.1.2), this strain was chosen for bioassay-guided isolation of active compounds.
In order to pursuit sufficient material for isolation and structure elucidation of
active metabolites, this strain was cultured in large scale as described in 2.5.3. The
biomass and cultivation medium were separated by centrifugation and filtration (see
2.5.3). The lyophilized biomass was then extracted with n-hexane, methanol, and
water, respectively as described in 2.6.1 to yield three extracts (n-hexane, methanol,
and water extracts) and the microscopically cell-free cultivation medium was
extracted with ethyl acetate as described in 2.6.2 to afford ethyl acetate extract. All
these extracts were tested for antimicrobial activity against Gram-positive and Gram-
negative bacteria and yeast Candida maltosa. It is interesting that among of all
extracts obtained from large scale culture, all extracts prepared from biomass
exhibited no activity against all test microorganisms while only the ethyl acetate
extract from the microscopically cell-free cultivation medium exhibited very strong
activity against all test microorganisms (see table 3-23). Thus, this active ethyl acetate
extract was chosen for further bioassay-guided isolation.
Table 3-23: Antibacterial activity of extracts from Anabaena sp. cultivated in large scale
Diameter of inhibition zone (mm)1
B.subtilic S.aureus E.coli C. maltosa
Ethyl acetate ext. from growth medium 15.0 16.0 24.0 16.0
Methanol ext. from biomass 02 0 0 0
n-hexane ext. from biomass 0 0 0 0
Water ext. from biomass 0 0 0 0
Ext. =extract; 1Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect.
3.5.1 Purification of EtOAc extract using semi-preparative reversed-phase
HPLC
Based on the result of the HPLC analysis, separation of the active crude
ethyl acetate extract by reversed-phase HPLC was done. Altogether, 12 mg of ethyl

Chapter III Results
95
acetate extract obtained from culture medium was purified with a step gradient of
CH3CN/H2O as mobile phase described in table 2-5. The collection of the separated
peaks was controlled at 238 nm and 254 nm. Seven peaks at retention times of 12.75,
15.76, 17.71, 18.33, 19.32, 20.77, and 22.77 min were collected respectively to yield
7 fractions (see fig.3-29). All these fractions were tested for antimicrobial activity
(table 3-24, fig.3-30)
f irst
AF1
AF3
AF5
AF6
AF2
AF4
f irst
Figure 3-29: Semi-preparative RP HPLC chromatogram of the crude EtOAc extract from culture medium (mobile phase see table 2-5), 1mg/50 µL/injection, detection at 238 nm, column Synergi POLAR-RP 80A (250×10 mm, 4 micron), flow rate 3 mL/min.
Table 3-24: Separation of EtOAc extract from Anabaena sp.culture medium by semi-preparative reversed-phase HPLC and antibacterial activity of the fractions to E. coli
Yield Fractions Retention
time (mg) % recovered
Dose (µg/disc) Diameter of inhibition
zone (mm)1
first 12.75 0.3 2.50 200 02
AF1 15.76 0.6 5.00 200 0
AF2 17.71 0.2 1.67 200 0
AF3 18.33 0.4 3.33 200 0
AF4 19.32 0.3 2.50 200 0
AF5 20.77 0.2 1.67 200 0
AF6 22.77 1.3 10.83 200 20.0
Total 3.3 27.50
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect

Chapter III Results
96
Figure 3-30: Agar diffusion test of 7 fractions obtained from ethyl acetate extract of culture medium of Anabaena sp. Test organism E.coli with c=0.2mg/disc
Because only fraction AF6 exhibited antimicrobial activity, this fraction was
used for structure elucidation.
3.5.2 Structure elucidation of fraction AF6 (compound 8)
Fraction AF6 was found to be the the pure substance flourensadiol (see fig.3-
31)
Figure 3-31: Flourensadiol, molecular formula C15H26O2
The HR-ESI-MS showed a sodiated molecular ion at m/z 261.1825 that was
compatible with the molecular formula C15H26O2. The 2D COSY spectrum allowed
the unambiguous identification of two fragments in the molecule, namely
CH3.CH.CH2.CH2 and CH2.CH.CH.CH, together with signals of an isolated CH2OH
and two singlet CH3 groups. The 13C and DEPT-135 NMR spectra indicate the
presence of 15 carbon atoms consisting of 3 methyl, 5 methylene, 5 methine and 2
quaternary carbons. A HMQC spectrum allowed correlation of these with the system

Chapter III Results
97
identified in the COSY spectra and identified a further complex methylene system and
methine system (see table 3-25). Two and three through-bond correlations in the
long-range 2D HMBC spectrum identified two systems incorporating the singlet
methyl groups and the isolated CH2OH group as CH3.C*(CH2OH) and CH3.C*(OH)
where C* are quaternary carbons (see table 3-25).
At this stage the Chapman and Hall data bank was searched using the
molecular formula and the characteristic CH3.C*(CH2OH) fragment. Thirty one
compounds were found of which only one, Flourensadiol (Murakami et al., 2001 and
Kingston et al., 1975) was compatible with the other fragments found in the molecule.
Further detailed inspection of the COSY and HMBC spectra confirmed that the
structure deduced from the NMR data were indeed compatible with the structure of
fluorensadiol originally determined by X-ray crystallography (Pettersen et al., 1975).
Characteristic NOE’s observed in the 2D ROESY spectrum (see table 3-25)
confirmed the relative stereochemistry was identical with the structure reported
previously.
We report the complete NMR data for the first time in the table 3-25.
Table 3-25: NMR data of AF6 (Flourensadiol) in trifluoroethanol-d2/H2O (1:1)
Carbon
No.
1H (ppm)
Multiplicity
J (Hz) 13C (ppm)
Multiplicity
HMBC (H—>C) ROESY
1 77.4 s 2 A 1.77 m
B 1.64 m 38.4 t
C1, C8
3 A 1.78 m B 1.47 ddd
14.3,11.8, 11.8
20.0 t C1 C2, C4, C5/C6, C1
4 0.83 ddd 11.8, 9.4, 5.8 29.8 d C5/C6, C13 5 25.0 s 6 0.38 dd 9.3, 9.3 25.1 d C4, C5, C3, C11,
C13, C14 H4, H7,
H9B, H10B, H12, H13
7 1.87 m 40.8 d C1 8 1.87 m 59.1 d 9 A 1.70 m
B 1.59 m 26.4 t
C8
10 A 1.82 m B 1.30 m
29.6 t C9
11 2.00 m 39.8 d --- 12 0.96 d 6.8 16.8 q 13 1.11 s 24.2 q C5, C4, C6, C14 14 A 3.77 d
B 3.71 d 11.6 11.6
65.3 t C5/C6, C4, C13 C5/C6, C4, C13
H7, H3B, H13
15 1.17 s 32.1 q C1, C2, C8 H2A, H2B,H8, H9A

Chapter III Results
98
3. 6 Chemical investigation of Nostoc sp.
The lyophilized biomass obtained from large scale culture (see 2.5.3) of
Nostoc sp. was extracted with n-hexane, methanol, and water, respectively to give
three extracts. Of three extracts, methanol extract exhibited the strongest activity
against Gram-positive bacteria (B. subtilic and S.aureus) and Gram-negative bacteria
(E.coli) and was therefore chosen for bioassay-guided isolation of antibacterial
metabolites.
3.6.1 Fractionation of methanol extract obtained from biomass using silica gel
column
760 mg of MeOH extract was first fractionated on silica gel column eluting
with a step gradient of n-hexane/EtOAc/MeOH/H2O (see 2.8.5) to yield 5 fractions
which were evaluated for their antibacterial activities against S. aureus using agar
diffusion assay (see table 3- 26).
Table 3-26: Fractionation of methanol extract from Nostoc sp. biomass by silical gel chromatography and antibacterial activity of fractions to S. aureus
Yield
Fractions
Mobile phase (mg) % recovered
Dose
(mg/disc)
Diameter of IZ
(mm)1
NFI n-hexane/EtOAc (90:10) 20.0 2.63 1.0 0.02
NFII n-hexane/EtOAc (40:60) 93.5 12.30 1.0 6.5
NFIII n-hexane/EtOAc (20:80) 36.1 4.75 1.0 0.0
NFIV MeOH 363.6 47.84 1.0 10.0
NFV MeOH/H2O (95:5) 26.3 3.46 1.0 0.0
Total 539.5 70.98
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect
Because fraction NFIV showed the strongest activity, this fraction was
further separated.
3.6.2 Separation of the acitve fraction NFIV using RP C18 column
chromatography
360 mg of the active fraction NFIV was subjected to C18 column eluting with
three solvent systems (see 2.8.5) to afford three fractions which were evaluated for

Chapter III Results
99
their antibacterial activities against S. aureus using agar diffusion assay (see table 3-
27).
Table 3-27: Fractionation of NFIV from Nostoc sp. biomass by RP C18
chromatography and antibacterial activity of fractions to S. aureus
Yield
Fractions
Mobile phase (mg) % recovered
Dose
(mg/disc)
Diameter of IZ
(mm)1
NFIV-1 MeOH/H2O (90:10) 151.6 42.11 0.5 11.0
NFIV-2 MeOH 96.3 26.75 0.5 0.02
NFIV-3 DCM 42.8 11.89 0.5 0.0
Total 290.7 80.75
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect
Only fraction NFIV-1 exhibited antibacterial activity and was further purified.
3.6.3 Purification of the active fraction NFIV-1 using semi-preparative reversed-
phase HPLC
Final purification of the active fraction NFIV-1 (150 mg) was achieved by
repeated reversed-phase HPLC to afford 4 fractions. All obtained fractions were
evaluated for antibacterial activity against S. aureus using agar diffusion assay (see
fig.3-32, table 3-28)
NsF1
NsF2
NsF3 NsF4
Figure 3-32: Semi-preparative RP HPLC chromatogram of fraction NFIV-1 with mobile phase of MeOH/ water + 0.05% TFA (see table 2.6) and 1mg/50 µL/injection, detection at 238 nm, column
Synergi POLAR-RP 80A (250×10 mm, 4 micron), flow rate 3 mL/min.

Chapter III Results
100
Table 3-28: Fractionation of NF IV-1 from Nostoc sp. biomass by semi-preparative RP HPLC and antibacterial activity of fractions to S. aureus
Yield Fractions Retention
time (mg) % recovered
Dose (µg/disc) Diameter of inhibition
zone (mm)1
NsF1 4.73 25.4 16.93 500 0.02
NsF2 5.48 3.6 2.40 500 10.0
NsF3 6.26 3.5 2.33 500 0.0
NsF4 7.71 1.7 1.13 500 0.0
Total
1. Diameter of inhibition zone (mm) includes Ø disc (6mm), 2. no effect
Only fraction NsF2 showed antibacterial activity, this fraction was therefore
used for structure elucidation.
3.6.4 Structure elucidation of fraction NsF2
The low resolution ESI-MS of fraction NsF2 which exhibited antibacterial
activity against Staphylococcus aureus with diameter of inhibition zone of 10.0mm in
concentration of 500mg/dics showed signal at m/z 426 [M+H]+. The NMR and MS
characterization of compound in this fraction NsF2 is in progress.
3.7 Chemical investigation of Lyngbya majuscula
Chemical investigation of this strain using two methods resulted in the
isolation of the 3 cytotoxic compounds anhydrodebrommoaplysiatoxin,
debromoaplysiatoxin, and anhydroaplysiatoxin.
Method1: The methanol extract resulting from lyophilized biomass of
L.majuscula prepared as described in 2.8.6.1 was separated by repeated silica gel
chromatography followed by preparative TLC to afford 17-debromo-3,4-didehydro-3-
deoxy-aplysiatoxin {(anhydrodebrommoaplysiatoxin) , 1.8 mg} with a yield of
0.12% based on the mass of the crude extract.
Method 2: The methanol extract from lyophilized biomass of L.majuscula
prepared as described in 2.8.6.2 was separated by silica gel chromatography followed
by reversed-phase HPLC to afford debromoaplysiatoxin, anhydroaplysiatoxin (3.8 mg
and 2.5mg ) with a yield of 0.12% and 0.21% , respectively, based on the mass of
crude extract.

Chapter III Results
101
3.7.1 Isolation of cytotoxic compounds of methanol extract obtained from
biomass according to method 1
The lyophilized biomass of this strain was extracted with n-hexane, methanol,
and water, respectively to give three extracts. Because the methanol extract exhibited
considerable cytotoxic activity, it has been chosen for isolation of the cytotoxic
substances using bioassay-guided fractionation.
3.7.1.1 Fractionation of methanol extract by silica gel column chromatography
In the first purification step, silica gel column chromatography of the
methanol extract (620 mg) using a stepwise gradient of n-hexane/EtOAc/ MeOH (see
2.8.6.1) yielded 15 major fractions (F1 to F15). Based on the results of TLC analysis, 7
fractions of these 15 fractions were tested for their cytotoxic activity (see table 3-29
and fig.3-33)
Table 3-29: Fractionation of methanol extract from Lyngbya majuscula biomass by silica gel chromatography and cytotoxic activity of fractions against cell line 5637
Yield Fracti
ons
No of sub-
fractions pooled
Mobile phase
mg % recovered
IC50
µg/L
F1 1-20 n-hexane/EtOAc (60:40) 1.86 0.3 n.e
F2 21-35 n-hexane/EtOAc (60:40) 8.06 1.3 4.2
F3 36-60 n-hexane/EtOAc (60:40) 24.18 3.9 10.0
F4 61-70 n-hexane/EtOAc (60:40) 2.79 0.45 0.1
F5 71-154 n-hexane/EtOAc (40:60) 40.61 6.55 6.5
F6 155-216 n-hexane/EtOAc (40:60) 60.45 9.75 n.e
F7 217-240 100% EtOAc 19.22 3.1 23.3
F8 241-308 100% EtOAc 119.6 19.29 8.4
F9 309-423 EtOAc/ MeOH (60:40) 17.98 2.9 2.1
F10 424 EtOAc/MeOH (40:60) 0.31 0.05 n.e
F11 425-431 EtOAc/MeOH (40:60) 0.93 0.15 n.e
F12 432-434 EtOAc/MeOH (40:60) 33.17 5.35 n.e
F13 435-437 EtOAc/MeOH (40:60) 17.05 2.75 n.e
F14 438-556 EtOAc/MeOH (40:60) 171.12 27.60 n.e
F15 557-640 100% MeOH 99.51 16.05 n.e
Total 616.84 99.49
n.e. not estimated

Chapter III Results
102
Figure 3-33: Thin layer chromatogram of 15 fractions obtained from silica gel column eluted with a stepwise gradient of n-hexane/EtOAc/MeOH (method1). {Mobile phase: n-hexane/EtOAc/MeOH/CH3COOH (75+25+5+3); Detection: Vis after spraying with Anisaldehyde/sulfuric acid reagent}
Because fraction F8 eluting with 100% EtOAc exhibited a strong cytotoxic
activity against 5637 cells (bladder cancer cell line) and only one main spot in TLC
was detected, it was selected for further separation.
3.7.1.2 Separation of fraction F8 by silica gel column chromatography
A portion of fraction F8 (90 mg) was further purified by silica gel column
chromatography with a stepwise gradient from 60% n-hexane to 90%EtOAc to 100%
MeOH as mobile phase ( see 2.8.6.1) to give six main fractions which were tested for
their cytotoxic activity (see table 3-30).
Table 3-30: Separation of fraction F8 from Lyngbya majuscula biomass by silica gel chromatography and cytotoxic activity of fractions to 5637 cell line
Yield
Fractions
No of sub-
fractions
pooled
Mobile phase mg % recovered
IC50
µg/L
F8-1 1-112 n-hexane/EtOAc (40:60) 9.6 10.67 0.16
F8-2 113-148 n-hexane/EtOAc (10:90) 118 13.11 0. 029
F8-3 149-208 n-hexane/EtOAc (10:90) 31.21 34.68 < 0.05
F8-4 209-214 n-hexane/EtOAc (10:90) 2.9 3.22 < 0.05
F8-5 215-225 n-hexane/EtOAc (10:90) 8.4 9.33 < 0.05
F8-6 226-325 100%MeOH 20.09 22.32 < 0.05
Total 84.00 93.33
Of these six fractions, fraction F8-3 exhibited the strong cytotoxic activity and
the highest yield and therefore it was selected for further purification.

Chapter III Results
103
3.7.1.3 Purification of fraction F8-3 by preparative TLC
30.2 mg of fraction F8-3 was further purified by preparative TLC. The
chromatogram was developed with n-hexane/EtOAc/MeOH/CH3COOH (75:25:5:3),
five spots were scratched out to yield five fractions (see table 3-31).
Table 3-31: Separation of fraction F8-3 from L.majuscula biomass by preparative TLC
Yield
Fractions
Retention factor mg % recovered
F8-3-1 0.28 1.2 3.97
F8-3-2 0.22 3.1 10.26
F8-3-3 0.18 1.7 5.62
F8-3-4 0.12 2.0 6.62
F8-3-5 0.045 0.1 0.33
Total 8.1 26.8
All five fractions (F8-3-1 to F8-3-5) were examined by analytical TLC and
HPLC. The result of HPLC analysis showed that fraction F8-3-2 (3.1 mg) was pure
enough for structure elucidation by ESI-MS and NMR.
3.7.1.4 Structure elucidation of fraction F8-3-2 (compound 9)
Fraction F8-3-2 was found to be the pure compound, 17-debromo-3,4-
didehydro-3-deoxy-aplysiatoxin (anhydrodebromoaplysiatoxin), a known metabolite
(see fig.3-34)
O
O O
H3C
H3C
O
OH
CH3O
CH3
O
CH3
OH
CH3
CH3
O
1
2
3
45
6
7
8
910
1112
1314
1516
17
18
19
2021
2223
2526
24
27
2829
30
31
32
Figure 3-34: 17-debromo-3, 4didehydro-3-deoxy –aplysiatoxin, molecular formula C32 H46O9
The high resolution ESIMS in positive mode of compound 9 showed ions at
m/z 543.2947 [M+H-MeOH]+, m/z 575.3209 [M+H]+, m/z 597.3027 [M+Na]+, and
m/z 613.6773 [M+K]+ (see fig.3-35) and the molecular weight was deduced to be 574

Chapter III Results
104
Dalton compatible with the molecular formula C32 H46O9 (calculated 575.3215 for C32
H47O9).
[ M + H -M eOH ] +
[ M + H ] + [ M+ N a ] +
[ M+ K] +
Figure 3-35: ESI-MS spectrum of fraction F8-3-2 (compound 9)
In addition, the loss of methanol in the mass spectrum requires the presence of a
methoxy group in the structure and in comparison with the literature data (Nagai et
al., 1998) and based on 1H NMR spectrum (see table 3-32 and appendix 10a,b)
structure of compound 9 was finally confirmed.
Table 3-32: Comparison of 1H NMR data of compound 9 with reported data *
Position
Compound 9 *Anhydrodebromoaplysiatoxin
1δH
2δH (J in Hz)
2 3.05 (d) 3.32 (d)
3.05 (br. d, 13.4 ) 3.32 (br. d, 12.1)
8 1.73 (dd) 2.22 (dd)
1.73(ax, dd, 3.6 and 14.8) 2.22 (eq, dd, 2.7 and 14.8)
9 4.84 (q) 4.84 (q, 2.9, 2.9 and 3.0) 11 3.77 (dd) 3.77 (dd, 1.8 and 10.7) 15 3.99 (t) 3.99 (t, 6.4 and 6.7) 17 6.82 (d) 6.82 (br. d, 7.6) 18 7.15 (t) 7.15 (t, 7.8 and 7.8) 19 6.72 (ddd) 6.72 (ddd, 1.0, 2.5 and 8.0) 21 6.86 (t) 6.86 (t, 1.7 and 2.3) 22 0.83 (3H, d) 0.83 (3H, d, 6.0) 23 0.82 (3H, d) 0.82 (3H, d, 6.0) 24 0.95 (3H,s) 0.95 (3H, s) 25 0.82 (3H,s) 0.82 (3H, s) 26 1.59 (3H,s) 1.59 (3H, s) 28 2.76(dd) 2.76 (dd, 3.8 and 17.9)

Chapter III Results
105
2.72 (dd) 2.72 (dd, 10.6 and 17.7) 29 5.30 (dt) 5.30 (dt, 3.8, 3.8 and 10.4) 30 3.84 (m) 3.84 (m) 31 1.11 (3H, d) 1.11 (3H, d, 6.5) 32
(CH3O-) 3.17 (3H,s) 3.17 (3H,s)
* Nagai.et al.,1998 1. Spectra determined in CDCl3 2. Spectra determined in acetone-d6; data reported in ppm
3.7.2 Isolation of cytotoxic compounds of methanol extract obtained from
biomass according to method 2
3.7.2.1 Separation of methanol extract by silica gel column chromatography
To remove a portion of unwanted components in the methanol extract the
dried residue of lyophilized biomass was extracted with methanol solvent after initial
extraction with n-hexane and ethyl acetate solvent, respectively.
A portion of the methanol extract (240 mg) was applied onto a silica gel
column and eluted with a stepwise gradient starting with 75% n-hexane to 100%
EtOAc to 100% MeOH (see 2.8.6.2) to yield twenty major fractions (F1 to F22). Based
on the results of the TLC analysis, three fractions (F9, F10, and F17) were chosen for
testing cytotoxic activity against 5637 cell line (see fig3-36 and table 3-33).
Figure 3-36: Thin layer chromatogram of 22 fractions obtained from silica gel column eluted with a stepwise gradient of n-hexane/EtOAc/ MeOH (method 2); [Mobile phase: n-hexane/EtOAc/MeOH/CH3COOH (75:25:5:3); Detection: Vis after spraying Anisaldehyde/sulfuric acid reagent]

Chapter III Results
106
Table 3-33: Fractionation of methanol extract from Lyngbya majuscula biomass by silica gel chromatography and cytotoxic activity of fractions against cell line 5637
Yield Fractions No of sub
fractions pooled
Mobile phase
mg % recovered
IC50
µg/ml
F1 1-3 n-hexane/EtOAc (75:25) 4.4 1.83 n.e
F2 4-8 n-hexane/EtOAc (75:25) 11.0 4.58 n.e
F3 9-16 n-hexane/EtOAc (75:25) 4.8 2.0 n.e
F4 17-27 n-hexane/EtOAc (75:25) 17.6 7.3 n.e
F5 28-48 n-hexane/EtOAc (75:25) 25.7 10.7 n.e
F6 49-80 n-hexane/EtOAc (75:25) 16.9 7.04 n.e
F7 81-104 n-hexane/EtOAc (50:50) 6.3 2.63 n.e
F8 105-107 n-hexane/EtOAc (50:50) 5.3 2.21 n.e
F9 108 n-hexane/EtOAc (50:50) 1.8 0.75 5.5
F10 109-120 n-hexane/EtOAc (50:50) 15.3 6.38 6.3
F11 121-138 n-hexane/EtOAc (25:75) 4.4 1.83 n.e
F12 139-154 n-hexane/EtOAc (25:75) 1.9 0.79 n.e
F13 155-159 n-hexane/EtOAc (25:75) 5.5 2.9 n.e
F14 160 100% EtOAc 11.7 4.88 n.e
F15 161-173 EtOAc/MeOH (90:10) 12.3 5.13 n.e
F16 174-180 EtOAc/MeOH (90:10) 0.1 0.04 n.e
F17 181 EtOAc/MeOH (90:10) 0.7 0.29 0.95
F18 182-195 EtOAc/MeOH (90:10) 0.9 0.38 n.e
F19 196 EtOAc/MeOH (25:75) 4.0 1.67 n.e
F20 197 EtOAc/MeOH (50:50) 16.7 6.96 n.e
F21 198 EtOAc/MeOH (25:75) 15.8 6.58 n.e
F22 199 100% MeOH 38.9 16.20 n.e
Total 222 93.07
n.e. not estimated.
According to the results of cytotoxicity test, the fraction F10 eluting with 50%
n-hexane/EtOAc which exhibited strong cytotoxic activity and did not show so many
spots in TLC was analyzed by HPLC to isolate pure compounds. HPLC analysis of
fraction F10 revealed that further separation by semi preparative HPLC was possible.

Chapter III Results
107
3.7.2.2 Purification of fraction F10 using semi-preparative reversed-phase
HPLC
The active fraction F10 was analyzed by HPLC with DAD detection. At 210
nm the separation of fraction F10 into five substances was successful using a Synergi-
RP (80A) column and a solvent gradient from 20%-100% acetonitrile/water. Based on
the results of analytical HPLC, the mobile phase (acetonitrile/water) for semi
preparative HPLC was established (see table 2.7). To optimize separation process,
different sample amounts (200, 400, 500, 1000, 1500, 2000 µg/50 µL injection
volume) for application on the column and different flow rates (2, 3, 3.5, 4, 5
mL/min) were tested. For a successful separation a sample amount of 500 µg/50 µL
injection volume and a flow rate of 3 mL/min were used.
Altogether, 14.0 mg of fraction F10 were purified using the step gradient
described in 2.8.1.2 and the collection of the separated peaks was controlled at the
wave length of 210 nm. Five peaks F10-1, F10-2, F10-3, F10-4, and F10-5 were collected to
give 5 fractions. All these fractions were tested for their cytotoxic activity against
bladder cancer cell line 5637 (see fig.3-37 and table 3-34)
F10 -3
F1 0 -4
F1 0 -5
F10 -2
F1 0 - 1
Figure 3-37: Semi-preparative RP HPLC chromatogram of F10 (mobile phase see table 2.7) 500 µg/50 µL injection volume, detection at 210nm, column Synergi POLAR-RP 80A (250×10 mm, 4 micron), flow rate 3mL/min

Chapter III Results
108
Table 3-34 Separation of fraction F10 from Lyngbya majuscula biomass by semi-preparative RP HPLC and cytotoxic activity of fractions against cell line 5637
Yield
Fractions
Retention time mg % recovered
IC50 (ng/ml)
F10-1 16.33 1.0 7.14 550
F10-2 19.23 1.1 7.86 380
F10-3 19.69 5.3 37.86 86
F10-4 23.50 1.9 13.57 No activity
F10-5 36.08 1.5 10.71 40
Total 10.8 77.14
Because fractions F10-3 and F10-5 exhibited strong activity (IC50= 86ng/mL
and 40ng/mL, respectively), the structure of including compounds was elucidated by
ESI –MS and NMR.
3.7.2.3 Structure elucidation of fractions F10-3 and F10-5
3.7.2.3.1 Structure elucidation of fraction F10-3 (compound 10)
Fraction F10-3 was recognized as the pure compound debromoaplysiatoxin (see
fig.3-38)
O
O O
H3C
H3C
O
OH
CH3O
CH3
O
CH3
OH
CH3
CH3
OH
O
1
2
3
45
6
7
8
910
1112
1314
1516
17
18
19
2021
2223
2526
24
27
2829
30
31
32
Figure 3-38: Debromoaplysiatoxin, molecular formula C32H48O10
The identification of debromoaplysiatoxin (compound 10) was established by
direct comparison of our spectroscopic data, including 1H NMR, 13C NMR, and
HRMS in negative mode with those reported in the literature. 1H NMR, 13C NMR
data of compound 10 were consistent with literature values for the known metabolite
debromoaplysiatoxin (Nagai et al., 1997) (see table 3-35 and appendix 11 a, b)

Chapter III Results
109
Table 3-35: Comparison of 13C NMR and 1H NMR data of compound 10 with literature values of *Debromoaplysiatoxin
Position
Compound
10
*Debromo-
aplysiatoxin
Compound
10
*Debromo-
aplysiatoxin 1
δC 2δC
3δH
4δH (J in Hz)
1 169.19 169.2 (0) - - 2 46.91 46.9 (2) 2.52 (β, d)
2.76 (α,d) 2.52 (β, d, 12.7) 2.76 (α, d, 12.5)
3 98.81 98.8 (0) - - 4 35.68 35.7 (1) 1.86 ( m) 1.86 (m) 5 41.16 41.1 (2) 1.62 (ax, t)
1.05 (eq, dd) 1.62 (ax, t, 13.1 and 13.1)
1.05 (eq, dd, 3.6 and 13.4)
6 38.98 39.0 (0) - - 7 100.85 100.8 (0) - - 8 33.66 33.6 (2) 1.71( ax, dd)
2.68 (eq, dd) 1.71 (ax, dd, 3.6 and 14.8) 2.68 (eq, dd, 3.0 and 14.7)
9 73.21 73.2 (1) 5.23 (m) - 10 35.42 35.4 (1) 1.71 (m) 1.71 ( m) 11 69.88 69.8 (1) 3.93 (dd) 3.93 (dd, 2.1and 10.9) 12 34.24 34.2 (1) 1.52 (m) 1.52 (m) 13 31.24 31.2 (2) 1.31 (m)
1.39 (m) 1.31 (m) 1.39 (m)
14 36.12 36.1 (2) 1.64 (m) 1.97 (m)
1.63 (m) 1.97 (m)
15 85.83 85.8 (1) 4.0 (t) 4.0 (t, 6.5 and 6.5) 16 145.93 145.9 (0) - -
17 119.34 119.3 (1) 6.84 (dt) 6.84 (dt, 1,1 and 7.9) 18 129.79 129.8 (1) 7.13 (t) 7.13 (t,7.8 and 7.8) 19 114.98 115.0 (1) 6.71 (ddd) 6.71 (dt,1,1 and 7.9) 20 158.34 158.3 (0) - - 21 114.67 114.6 (1) 6.92 (m) 6.92 (t,1 and 1) 22 13.52 13.6 (3) 0.79 (d) 0.79 (d,6.8) 23 13.04 13.0 (3) 0.71 (d) 0.71 (d,6.9) 24 26.79 26.8 (3) 0.83 (s) 0.83 (s) 25 23.61 23.6 (3) 0.8 (s) 0.8 (s) 26 16.48 16.5 (3) 0.86 (d) 0.86 (d,6.8) 27 170.36 170.4 (0) - - 28 34.69 34.6 (2) 2.92 (α, dd)
2.88 (β, m) 2.92 (α, dd, 11.1 and
18.2) 2.87 (β, dd, 2.8 and 18.1)
29 74.30 74.3 (1) 2.23 (m) 5.23 (m) 30 67.08 67.0 (1) 4.03 (m) 4.03 (m) 31 17.72 17.7 (3) 1.12 (d) 1.12 (d, 6.4) 32 56.60 56.6 (3) - 3.17 (s)
* Nagai et al.,1997 1. Spectra determined in CDCl3; data reported in ppm 2. Spectra determined in acetone-d6; data reported in ppm. Carbon types determined by a DEPT experiment and reported as 0 (quaternary), 1(CH), 2(CH2), or 3(CH3) 3. Spectra determined in CDCL3, 600 MHz; data reported in ppm 4. Spectra determined in acetone-d6, 500 MHz; data reported in ppm
In addition, the HRMS in negative mode of compound 10 showed a
molecular ion peak at m/z [M-H+HCl]- 627.2942 (see fig.3-39) and the molecular

Chapter III Results
110
weight was deduced to be 592 Dalton compatible with the molecular formula
C32H48O10.
[ M - H + H C l ] -
Fgure 3-39: UV and ESI-MS spectrum of compound 10
3.7.2.3.2 Structure elucidation of fraction F10-5 (compound 11)
Fraction F10-5 was identified as the pure compound 3,4-didehydro-3-deoxy-
aplysiatoxin (figure 3-40)
O
O O
H3C
H3C
O
OH
CH3O
CH3
O
CH3
OH
CH3
CH3
O
1
2
3
45
6
7
8
910
1112
1314
1516
17
18
19
2021
2223
2526
24
27
2829
30
31
32
Br
Figure 3-40: 3,4-didehydro-3-deoxy-aplysiatoxin, molecular formula C32H45BrO9
The high resolution ESIMS in positive mode of compound 11 showed two
peaks as a molecular ion peak at m/z 653.2329 [M]+ and an isotope ion peak at m/z
655. 2311 [M+2]+ which were very similar in height (see fig. 3-41) indicating that
compound 11 contains a bromine atom and the molecular weight was deduced to be
653 Dalton compatible with the molecular formula C32 H45 BrO9 (calculated 653.2320
for C32 H46BrO9).

Chapter III Results
111
[ M ] + [ M + 2 ] +
[ M + 2 ] +
Figure 3-41: UV and ESI-MS spectrum of compound 11
1H NMR data of compound 11 were fully consistent with literature values for
the known metabolite 3,4-didehydro-3-deoxy- aplysiatoxin (Moore et al., 1984) (see
table 3-36 and appendix 12a,b).
Table 3-36: Comparison of 1H NMR data of compound 11 with reported data *
Position
Compound 11 *3,4-didehydro-3-deoxy-
aplysiatoxin
1δH
2δH
2 3.070 (dm)
3.342 (d)
3.070 (br dm)
3.342 (d)
5 2.185 (dm) 2.185 (br dm)
11 3.805 (dd) 3.805 (dd)
15 4.447 (dd) 4.447 (dd)
18 7.357 (dd) 7.357 (dd)
19 6.715 (d) 6.715 (d)
21 7.008 (d) 7.008 (d)
22 0.845 (d) 0.845 (d)
23 0.856 (d) 0.856 (d)
24 0.956 (s) 0.956 (s)
25 0.829 (s) 0.829 (s)
28 2.739 (dd) 2.739 (dd)

Chapter III Results
112
2.781 (dd) 2.781 (dd)
29 5.357 (dt) 5.357 (dt)
30 3.854 (qd)
4.07 (d)
3.854 (qd)
4.07 (d)
OCH3 3.210 (s) 3.210 (s)
*Moore et al., 1984 1. Spectra determined in CDCl3; data reported in ppm 2. Spectra determined in acetone-d6; data reported in ppm
3.7.3 Fatty acid analysis
The mixture of compounds in n-hexane extract of L. majuscula was separated
by gas chromatography and the summary of fatty acids in this extract was shown in
table 3-37 and appendix 13.
Table 3-37: Fatty acids analyzed as methyl ester in n-hexane extract
ret.
time [min]
trivial name main mass spectral
fragment (m/z)
percent
(%)
match
(%)
28.498 Myristic acid (C14:0) 285, 201, 117, 73, 43 0.925 71.5
29.888 Pentadecanoic acid (C15:0) 299, 201, 117, 73, 40 0.663 74.8
32.257 Palmitic acid (C16:0) 313, 201, 117, 73, 47 3.64 88.0
35.072 Linoleic acid (C18:2) 337,262,73 0.391 73.3
35.531 Stearic acid (C18:0) 339, 222, 117, 73, 55 22.748 84.8
38.788 Arachidic acid (C20:0) 367, 292, 185,117, 73 0.606 75.4
48.997 Cholesterol 458, 368, 392, 247,
129, 73
1.062 83.1
ret. time = retention time; m/z = the mass of an ion divided by the electrical charge of the ion
It was shown, that in n-hexane extract of Lyngbya majuscula the main fatty
acids were stearic acid and palmitic acid. Beside this, cholesterol, the unsaturated
linoleic acid and other fatty acids were also found.

Chapter IV Discussion
113
4 Discussion
4.1 Screening of crude extracts for antibacterial activity
4.1.1 Selection of antibiotic screening and cyanobacterial strains
Antibiotics were considered to be "miracle drugs" when they first became
available half a century ago, but their popularity rapidly led to overuse (Saleem et al.,
2010). Over the last decade, it has become clear that antimicrobial drugs are losing
their effectiveness due to the evolution of pathogen resistance, as resistance to
antibiotics in bacterial population increased dramatically with time and usage of
antimicrobial drugs. There is therefore a continuing need to explore, search for and
develop newer and more effective broad spectrum antimicrobial agents.
Against the growing problem of antibiotic-resistant bacteria, alternative
antimicrobial drugs sources which are nontoxic/ less toxic to human and without side
effects for human and for environment must be found.
Natural products are both fundamental sources of new chemical diverse and
integral components of today’pharmaceutical compendium (Saleem et al., 2010) and
natural antimicrobials will undoubtedly have an important role in protecting against
infection. This new direction in research has been the subject of many studies on
antimicrobial effects of various organisms including cyanobacteria. The medicinal
qualities of cyanobacteria were first appreciated as early as 1500 BC, when Nostoc
species were used to treat gout, fistula and several forms of cancer. Yet, not much
attention was paid till the turn of the century when during 1990, workers at University
of Hawaii, Oregon State had begun to screen extracts of cyanobacteria, mostly strains
of Microcystis and Anabaena spp. for various biological activities. It was reported that
nearly 4000 strains of freshwater and marine cyanobacteria were screened inferring
that cyanobacteria are a rich source of potentially useful natural products (6% having
anticancer, antiproliferative activity). Later, several screening processes were initiated
with important targets such as antibacterial, antifungal, anti-AIDS, anticancer and
other activities. Screening of cyanobacteria for antibiotics has opened a new horizon
for discovering new drugs. Numerous screening programs have revealed the potential
of cyanobacteria in the production of novel antimicrobial compounds (Schlegel et al.,
1999; Mian et al., 2003; Soltani et al., 2005; Pawar &Puranik, 2008). Various strains
of cyanobacteria are known to produce intracellular and extracellular metabolites with

Chapter IV Discussion
114
antibacterial activity (Falch et al., 1995; Jaki et al., 1999a, 2000a,b; Harvey, 2000;
Mundt et al., 2001; Volk &Furkert, 2006; Asthana et al., 2006; Rao et al., 2007;
Kaushik &Chauhan, 2008). Many studies have assessed the antibacterial activity of
some cyanobacteria and their extracts ( Kreitlow et al., 1999; Schlegel et al., 1999;
Mian et al., 2003; Ghasemi et al., 2003; Mundt et al., 2003; Ghasemi et al., 2004;
Soltani et al., 2005; Volk &Furkert, 2006; Asthana et al., 2006; Taton et al., 2006;
Abedin &Taha, 2008; Martins et al., 2008; Abdel-Raouf & Ibraheem, 2008; Patil et
al., 2009).
There are numerous reports on biological active compounds isolated from
cyanobacteria living in both freshwater and marine environments (e.g. Østensvik et
al., 1998; Harada, 2004; Fassanito et al., 2005; Tan, 2007). But little work has done to
screen cyanobacteria isolated from rice, cotton and coffee fields with regard to their
production of antibacterial substances. In one study, the culture media of
cyanobacteria belonging to Nostocaceae, Microchaetaceae and Scytonematacaea
isolated from the Argentinian paddy-fields, were found to be active against S. aureus
(De Caire et al., 1993). Investigations of another group showed that cyanobacteria
from paddy fields of northern Thailand produce bioactive substances with antibiotic
activity against B. subtilis (Chetsumon et al., 1993). Also, Soltani et al., 2005
isolated 76 cyanobacterial strains from Iranian paddy fields. 22.4% of them (17
cyanobacteria belonging to Stigonemataceae, Nostocaceae, Oscillatoriaceae, and
Chrococcaceae) exhibited antimicrobial effects and growth of B. subtilis PTCC 1204,
Staphylococcus epidermidis PTCC 1114, E. coli PTCC 1047, and Salmonella typhi
PTCC 1108 was inhibited by 12, 14, 8, and 2 strains of cyanobacteria, respectively.
Only few reports publish data about screening of Vietnamese cyanobacteria
with regard to their production of bioactive compounds (Bui et al., 2007), and none
relate to antibacterial screening and new biological active compounds of terrestrial
cyanobacteria from South Vietnam. Thus, screening of 12 cyanobacterial strains
isolated from rice, cotton and coffee fields of Dak Lak province located in South
Vietnam for their antibacterial activities is among the first studies done for assessment
of antibacterial activity of Vietnamese terrestrial cyanobacteria with the objective of
finding new antibacterial compounds which could serve as a source of new lead
structures for development of antibacterial drugs or chemical leads useful in
facilitating the development of new therapeutic or commercial agents in the future.

Chapter IV Discussion
115
12 cyanobacterial strains belonging to 6 genera isolated from rice, cotton and
coffee fields were chosen for this study to provide a broad spectrum of different
cyanobacterial species. Many of the selected genera are known for producing
biologically active compounds however, they are not well documented in literature.
4.1.2 Cultivation and extraction
The BG-11 medium was selected as the primary cultivation medium as it
was designed for culturing cyanobacteria (Watanabe, 2005) and has been used for
culture 12 selected cyanobacterial strains successfully.
The temperature used for cyanobacterial cultures in this work was room
temperature (20±20C), this temperature is consistent with a general rule that the
optimum temperature for cyanobacteria is 15-200C (Castenholz, 1988; Andersen,
2005).
The light source used for cyanobacterial culture in this work were cool
fluorescence lamps because the spectral range of light absorbed by cyanobacteria
requires the use of fluorescent light (i.e. cool-white, warm-white, daylight), other
light sources have a great proportion of the output in the far red and near-infrared
regions which are not available to oxygenic phototrophs.
The growth period of the samples lasted 4-6 weeks to ensure that the
majority of cultures were in stationary phase and therefore most of likely producing
secondary metabolites (Lincoln et al., 1996).
The cultures were harvested by centrifugation followed by filtration with
filter paper to separate cells (biomass) and growth media. The harvested biomasses
were freeze dried and extracted. In order to separate unknown chemical compounds
from the totality of cyanobacterial metabolites, procedures modified from methods of
plant natural products isolation (Cannell, 1998) were used. The dried biomasses of
cyanobacteria were successively extracted with solvents of increasing polarity: n-
hexane, methanol, and water. By modifying the extraction method for extracellular
metabolites from bacteria (Cannell, 1998), the harvested growth media were extracted
with ethyl acetate by liquid- liquid extraction to get ethyl acetate extracts. All
extracts were tested in vitro for their antibacterial activity.

Chapter IV Discussion
116
4.1.3 Antibacterial activity
Our data revealed that of 48 extracts, 23 (47.92%; 3 n-hexane, 11 MeOH,
and 9 EtOAc extracts) showed activity against the Gram-positive bacterium B.subtilis,
22 (45.83%; 3 n-hexane, 11 MeOH, and 8 EtOAc extracts) exhibited activity against
the Gram-positive bacterium S. aureus, 11 (22.92%; 1 n-hexane, 4 MeOH, and 6
EtOAc extracts) inhibited the growth of the Gram-negative bacterium E. coli, 3
(MeOH extracts) showed effects on the growth of the Gram-negative bacterium P.
aeruginosa, but these effects did not result in complete inhibition, and none of the
water extracts was active against test bacteria. These results indicate that the extracts
contained different antibacterial substances and reflected the variety of secondary
metabolites and are also in agreement with results in the literature. For example, Jaki
et al., 1999b found that of 86 extracts of cyanobacteria, 14 (16.3%; 9 DCM/MeOH
2:1 and 5 MeOH/H2O 7:3 extracts) showed activity against Gram-positive bacteria. 5
extracts (5.8%; 3 DCM/MeOH 2:1 and 2 MeOH/H2O 7:3 extracts) exhibited activity
against Gram-negative bacteria. Similarly, Falch et al. 1995 reported that the
petroleum ether, dichloromethane, ethyl acetate, methanol and 80% aqueous methanol
or water extracts of cyanobacteria showed different antibacterial effects in
bioautographic assays with B. subtilis and E. coli. Soltani et al., 2005 reported that
the petroleum ether, methanol, and aqueous, extracts of cyanobacteria showed
antimicrobial activity.
The results of this study showed that the growth of Gram-positive bacteria
was more inhibited in comparison with Gram-negative bacteria, this was in agreement
with the results of previous studies (e.g. Jaki et al., 1999b; Mundt et al., 2001; Mian et
al., 2003; Ghasemi et al., 2003; Soltani et al., 2005; Taton et al., 2006, Asthana et
al., 2006, Volk &Furkert, 2006; Martins et al., 2008). This finding may be related to
the fact that cyanobacteria possess features familiar to Gram-negative bacteria
(Dahms et al., 2006) and the most Gram-negative bacteria are resistant to toxic agents
in the environment due to the barrier of lipopolysaccharides of their outer membrane
(Dixon et al., 2004; Martins et al., 2008). The difference in toxicity against Gram-
positive and Gram-negative bacteria can also indicate that the mechanism of toxicity
is different in the two types of cells caused by different permeability to the
cyanobacterial compounds (Martins et al., 2008). In addition, in ecological
consideration, cyanobacteria possess morphology of Gram-negative bacteria; the fact
that the bioactive cyanobacterial metabolites are effective more strongly against

Chapter IV Discussion
117
Gram-positive bacteria approves the idea that the antibiotic substances are secondary
metabolites produced for defense purpose in cyanobacterial life (Piccardi et al., 2000;
Bhadury &Wright, 2004).
In this study, all investigated cyanobacteria (12/12) showed an antibacterial
activity to at least one of the test organisms while in the literature17 of 76 investigated
cyanobacterial strains (22.4%) exhibited antimicrobial effects (Soltani et al., 2005).
Thus our results provide evidence to further support the use of cyanobacteria in drug
discovery efforts for antibiotic lead compounds and it points out the necessity of
exploring cyanobacteria of local habitats as potentially excellent sources of these
compounds.
As mentioned in 1.2, most of bioactive compounds isolated from
cyanobacteria belong to the groups of peptides (e.g. cyclic depsipeptides, cyclic
peptides, and lipopeptides), fatty acids, polyketides, alkaloids, amides, terpenoids,
lactones, pyrroles, and others. From our screening, because different activities were
observed in extracts obtained with different organic solvents we can suggest that
compounds with different polarities are involved. Especially, antibacterial activities
were found mostly in methanol and ethyl acetate extracts, therefore substances of low
polarity to middle polarity such as fatty acid, polyketides, peptides, alkaloids, and
terpernoids were expected.
Of the twelve investigated cyanobacteria, all crude extracts of Westiellopsis
sp.VN and the crude ethyl acetate extract of Anabaena sp. showed very strong
antibacterial activity against Gram-positive and Gram-negative bacteria, as well as
the crude methanol extracts obtained from Calothrix javanica, Scytonema ocellatum,
Nostoc sp. showed strong antibacterial activity (see 3.1.2). Therefore these strains
were selected for chemical investigation with an emphasis on the isolation and
structure elucidation of antimicrobial secondary metabolites.
4.2 Chemical investigation and culture optimization of Westiellopsis
sp. VN
4.2.1 Selection of Westiellopsis sp.VN
The strain Westiellopsis sp.VN isolated from rice field soil of Dak Lak
province in Southern Vietnam was described on morphological characteristics and

Chapter IV Discussion
118
identified based on its partial 16S rRNA gene sequence and molecular-phylogenetic
tree constructed based on 16S rRNA gene sequences of this cyanobacterial strain and
11 other cyanobacterial strains belong to genera Westiellopsis and Fischerella in
GenBank (Ho et al., 2005a; Ho, 2007). It is interesting to note that in Vietnam,
species of genus Westiellopsis have not been described yet; therefore Westiellopsis sp.
VN strain was described for the first time by Ho et al., 2005a.
Up to now, there is no literature available on chemical investigations of
Vietnamese Westiellopsis genus and the results of screening demonstrated that all the
crude extracts of Westiellopsis sp. VN exhibited very strong antibacterial activity
against Gram-positive and Gram-negative bacteria (see 3.1.2), as well as yeast
Candida maltosa SBUG 700 {(IZ of 16.5mm including diameter of disc (6mm) with
C=2mg/disc in agar diffusion assay}. Thus, the Westiellopsis sp.VN strain was first
selected for the detailed investigation with an emphasis on the isolation and structure
elucidation of antimicrobial secondary metabolites.
4.2.2 Active intracellular metabolites of Westiellopsis sp.VN strain
Branched filamentous cyanobacteria belonging to the order Stigonematales
are known to produce antibacterial, antifungal, antialgal and cytotoxic compounds,
with diverse chemical structures. To date, examination of the genus Fischerella,
Hapalosiphon, and Westiella resulted in the isolation of isonitrile-containing indole
alkaloids such as hapalindoles (Moore et al., 1984a; Huber et al., 1998), ambiguines
isonitriles (Smitka et al., 1992; Park et al., 1992; Huber et al., 1998; Raveh &Carmeli,
2007, Mo et al., 2009), fischerindoles (Park et al., 1992), and welwitindolinones
(Stratmann et al., 1994). However, only few biologically active compounds were
identified from the genus Westiellopsis, e.g. westiellamide, a bistratamide-related
cyclic peptide from terrestrial cynobacterium Westiellopsis prolifica Janet exhibited
cytotoxic activity against KB and LoVo cell lines at 2 µg/mL, but not solid tumor
selectivity in the Corbett assay (Prinsep et al., 1992). This compound appears to be
identical with a bistratamide-type compound isolated from the ascidian Lissoclinum
bistratum, suggesting that cyanophyte symbionts in Lissoclinum bistratum are
responsible for synthesis of the bistratamides (Patterson et al., 1992).
The chemical investigation of the methanol extract prepared from dry
biomass of Westiellopsis sp.VN using bioassay-guided isolation led to the isolation

Chapter IV Discussion
119
and/or identification of the six intracellular compounds ambiguine D isonitrile,
ambiguine B isonitrile, dechloro-ambiguine B isonitrile, and fischerellin A as well as
hydroxy-eicosatetraenoic acid and methoxy-nonadecadienoic acid. Identification of
these active compounds was established by direct comparison of our spectroscopic
data, including 1H NMR and HR-ESI-MS with those reported in the literature.
Ambiguine D isonitrile was first isolated from Westiellopsis prolifica EN-3-1
and identified conclusively by 1H NMR and TLC analysis. This compound displayed
moderate antifungal activity to Candida albicans, Trichophyton mentagrophytes, and
Aspergillus fumigatus with MIC values of 1.25, >80.0, and > 80.0 µg/mL,
respectively (Smitka et al., 1992). Ambiguine D isonitrile was also isolated from
Fischerella sp. (Raveh &Carmeli, 2007) and only in trace amounts detected by LC-
MS in Fischerella ambigua UTEX 1903 (Mo et al., 2009). In our experiments,
ambiguine D isonitrile was isolated from Westiellopsis sp.VN and this compound
exhibited antibacterial activity against S. aureus with diameter of inhibition zone of
28.0mm in concentration of 200mg/dics (see table 3-4).
Ambiguine B isonitrile was isolated from the terrestrial cyanobacterium
Fischerella ambigua UTEX 1903. This compound showed moderate antifungal
activity to Candida albicans, Trichophyton mentagrophytes, and Aspergillus
fumigatus with MIC values of 1.25, >80.0, 20.0 µg/mL, respectively (Smitka et al.,
1992) and moderate antimicrobial activity to Bacillus anthracis, Staphylococcus
aureus, Mycobacterium smegmatis, Candida albicans with MIC values of 3.7, 10.9,
27.8, 1.7 µM, respectively, as well as cytotoxic activity to Vero cells with IC50 value
of 58.6 µM (Mo et al., 2009). Ambiguine B isonitrile was also isolated from terrestrial
cyanobacterium Hapalosiphon delicatulus (Huber et al., 1998) and Fischerella sp.
(Raveh & Carmeli, 2007). In this work, ambiguine B isonitrile was isolated from
Westiellopsis sp.VN and showed antibacterial activity against S. aureus with diameter
of inhibition zone of 8.0mm in concentration of 200mg/dics (see table 3-4).
Ambiguine C isonitrile was isolated from the terrestrial cyanobacterium
Fischerella ambigua UTEX 1903. This compound showed moderate antifungal
activity to Candida albicans, Trichophyton mentagrophytes, and Aspergillus
fumigatus with MIC values of 2.5, >80.0, >80.0 µg/mL, respectively (Smitka et al.,
1992) and moderate antibacterial activity to Mycobacterium tuberculosis, Bacillus
anthracis, Staphylococcus aureus, Mycobacterium smegmatis, Candida albicans with
MIC values of 7.0, 16.1, 7.4, 59.6, <1 µM, respectively, as well as cytotoxic activity

Chapter IV Discussion
120
to Vero cells with IC50 value of 78.3 µM (Mo et al., 2009). In our case, ambiguine C
isonitrile was observed in a mixture with other compounds of fraction WF1-5 which
showed antibacterial activity against S .aureus with diameter of inhibition zone of
10.0mm in concentration of 200mg/dics (see table 3-4).
Fischerellin A, a photosystem II-inhibiting allelochemical with antifungal and
herbicidal activity (Hagmann & Jüttner, 1996) was isolated from Fischerella
muscicola UTEX 1829. In our work this compound was observed also as a mixture in
fraction WF1-5 which showed antibacterial activity against S .aureus with IZ of 10.0
mm (see table 3-4).
Two derivatives of fatty acids, methoxy-eicosatetraenoic acid and hydroxy-
nonadecadienoic acid have been identified in fraction WF1-8. They were found as a
mixture in fraction WF1-8 which showed antibacterial activity against S.aureus with
diameter of inhibition zone of 10.0mm in concentration of 200mg/dics (see table 3-4).
However, in contrast to clear structure elucidation of the 4 compounds ambiguine B,
C, D isonitriles and fischerellin A, the chemical structure of the two derivatives of
methoxy-eicosatetraenoic and hydroxy-nonadecadienoic acid has not clearly
elucidated.
In summary, bioassay–guided fractionation of the methanol extract obtained
from biomass of our Westiellopsis sp. VN led to the isolation of ambiguine B,D
isonitriles and the identification of ambiguine C isonitrile and fischerellin A.
Furthermore, two derivatives of fatty acids methoxy-eicosatetraenoic acid and
hydroxy-nonadecadienoic acid have been identified. These compounds have not been
described as constituents of Westiellopsis sp. VN previously. Interestingly, up to the
present study, 15 ambiguine A-O isonitriles have been found in Westiellopsis prolifica
EN-3-1, Hapalosiphon delicatulus, Fischerella sp, Fischerella ambigua UTEX 1903
but not yet in Fischerella muscicola UTEX 1829, while fischerellin A only has been
isolated from Fischerella muscicola UTEX 1829 and other strain of genus Fischerella
so far. In our Westiellopsis sp VN ambiguine B, C, D isonitriles and fischerellin A
were detected.
Moore et al., 1987 proposed a common biogenesis of the hapalindoles,
ambiguines, fischerindoles, and welwitindolinones and this proposal has supported by
Strantman et al., 1994; Raveh & Carmeli, 2007; Van Wagoner et al., 2007;
Gademann & Portman, 2008; Mo et al., 2009. Several polycyclic
tryptophan/isoprenoid compounds have been reported from genera Fischerella,

Chapter IV Discussion
121
Hapalosiphon, Westiella, and Westiellopsis, order Stigonematales. The rich diversity
in their cyclic structures highlights the flexibility isoprenoid chemistry in forming
carbon-carbon bonds. Some of schemes presenting possible biogenetic relationships
among some of the dozens of reported compounds of the family Stigonemataceae
were published by Stratmann et al., 1994, Raveh & Carmeli, 2007; Van Wagoner et
al., 2007; Gademann & Portman, 2008. It has been suggested in literature that co-
occurrence of nonchlorinated and chlorinated ambiguine isonitriles in cyanobacteria
of the order Stigonematales may be due to some imperfection in the biosynthesis and
the resulting arrays of compounds have been proposed to provide an ecological
advantage (Raveh & Carmeli, 2007). In our study, the identification of the
nonchlorinated ambiguine C isonitrile together with the chlorinated ambiguine B, D
isonitriles found in Westiellopsis sp.VN, was therefore not surprising.
The Fischerellins (see fig. 4-1), previously isolated only from genus
Fischerella, are enediyne metabolites that showed activity against photosynthetic
microorganisms including cyanobacteria (Gross et al., 1991; Hagmann &Jüttner,
1996; Papke et al., 1997).
Figure 4-1: Fischerellins from cyanobacteria
The configuration of the fischerellin enediyne moiety (ene/yne/yne) is
topologically distinct from that seen in the more famous anticancer enediynes from
actinomycetes. An interesting difference between fischerellin A and B is the presence
of methyl branching on the side chain in the former, and absence of methylation but
extension of the side chain by one carbon in the latter. No biosynthetic studies have
been undertaken for the fischerellins until now (Van Wagoner et al., 2007).
It is interesting to note that occurrence of fischerellin A has not been
described for genus Westiellopsis, but the close relationship between the genera
Fischerella and Westiellopsis could allow a gene transfer between the organisms.
Otherwise it was not excluded, that genetic information for biosynthesis of

Chapter IV Discussion
122
fischerellins was repressed in Westiellopsis strains and can be activated by different
endogenic or exogenic factors.
4.2.3 Chemical composition of volatile extracellular compounds of Westiellopsis
sp.VN strain
Bioactive metabolites of cyanobacteria which have been isolated so far have
been mostly accumulated in the cyanobacterial biomass. Moreover, cyanobacteria
excrete various organic compounds into their environment and, until now, a couple of
biologically active compounds were also identified among these extracellular
metabolites, e.g. some antibacterial diterpenoids in Nostoc commune (Jaki et al.,
1999a, 2000a), antifungal peptides in Tolypotrix byssoidea (Jaki et al., 2001), and the
antialgal indol alkaloid norhamane (Volk &Furkert, 2006). In our investigations of
ethyl acetate extract of culture medium of Westiellopsis sp.VN we found a strong
antibacterial activity of this extract. Based on this we have developed isolation
procedure for the active compounds, excreted by Westiellopsis sp.VN strain into its
environment.
Ethyl acetate extract was extracted with different solvents and only methanol
fraction of this extract showed antibacterial activity. Microorganisms typically
produce an extracellular product in low concentration (<3%) (Gailliot, 1998) resulting
in small amount of methanol fraction. Separation of the components of this fraction
by HPLC was not successful, though different solvent gradients and conditions have
been used. Therefore GC-MS was used for identifying the volatile components.
Results of GC-MS revealed that this fraction contains different saturated fatty acids
with 14, 16, and 18 carbons, the unsaturated 11-cis-octadecanoic acid, naphthalene,
carbamic acid and 5-oxoproline.
In many studies, it has been shown that fatty acids and volatile components
are responsible for a broad spectrum of antibacterial activity and other activities
(Mundt et al., 2003; Ozdemir et al., 2004). Thus, the presence of identified
compounds in this methanol fraction is not surprising, however to verify whether only
these compounds are the cause for very strong antimicrobial activity of this methanol
fraction, further separation of this methanol fraction is necessary.

Chapter IV Discussion
123
4.2.4 Cultivation optimization of Westiellopsis sp.VN strain
Clearly, cyanobacteria produce a large number of biologically active
metabolites with potential pharmaceuticals but in general there are still some serious
obstacles to the development of drugs from cyanobacteria. The most obvious are their
slower growth rate in culture and lower biomass yield as well as the smaller quantities
of secondary metabolites produced in comparison with bacteria and fungi.
Additionally, a number of studies (Carmichael, 1986; Borowitzka, 1995) have
verified that the desired bioactive compounds may decline, alteration or be lost
entirely during culture, though reasons for this phenomenon are so far inexplicable.
Even when seemingly stable strains of drug –producing cyanobacteria are obtained
using conventional cloning techniques, drug production sometimes disappears on
repeated subculturing, especially if culture conditions are modified, such as anatoxin-
a toxicity in Anabaena flos-aquae NRC-44-1 disappeared when the medium was
changed from ASM-1 to the nitrate- richer BG 11; similarly, repeated subculturing of
Anabaena flos-aquae S-23-g, a strain that produces the neurotoxin anatoxin-d, with
ASM-1 followed by BG11 resulted in loss of neurotoxicity and expression of
hepatotoxicity similar to that observed in Microcystis (Carmichael, 1986). Thus, it is
of great importance to optimize the cultivation conditions and production of
metabolites in cyanobacteria.
Temperature during incubation period, pH of the culture medium, phosphate
concentration, length of incubation period, salinity, medium constituents, light
intensity are important factors influencing biomass and bioactive secondary
metabolite production (e.g. Repka et al., 2001; Griffiths & Saker, 2003; Ame et al.,
2003; Hirata et al., 2003; Noaman et al., 2004; Abu et al., 2007; Abedin & Taha,
2008; Imai et al., 2009). Carbon and nitrogen sources fed to the antimicrobial
agent producing cyanobacteria may exhibit significant roles in orienting the
secondary metabolic pathways (Sailer et al.,1993; Griffiths & Saker, 2003).
Medium studies and optimization methods are commonplace with respect to
increasing the yield or activity of a given product. Until now, there have been some
papers concerned with the optimization of growth rate and bioactive secondary
metabolite production, especially antibiotic production by cyanobacteria (Moore et
al., 1988; Bloor & England, 1991; Chetsumon et al., 1993; Noaman et al., 2004;
Panda et al., 2006; Silva & Silva, 2007). Such examples are:

Chapter IV Discussion
124
- The seeking out the optimum with respect to antibiotic production by Nostoc
muscorum was undertaken by Bloor &England, 1991 and the results of their
experiments showed that an increased level of nitrate and a decreased level of iron,
compared with the starting basal medium, promoted antibiotic activity. Furthermore
it was seen that growth was limited at lower iron concentrations (3 µM and 0 µM)
and it is likely that available iron in the growth medium represses biosynthesis of
enzymes that are necessary for antibiotic production. It is not yet clear whether iron
can inhibit antibiotic production after the organism has started production.
- Optimization of antibiotic production by the cyanobacterium Scytonema
sp.TISTR 8208 immobilized on polyurethane foam was studied by Chetsumon et al.,
1993. This study showed that the Scytonema sp.TISTR 8208 produced an antibiotic
with a broad spectrum in the post-exponential growth phase. Modification of the
composition of the BGA medium by adding 1.5 g L-1 NaNO3, increasing the
Fe2(SO4)3.x6H2O concentration to 0.025 g L-1, not adding NaCl, using an initial pH of
7.0, incubating at 350C, and at the light intensity of 90µmol photon m-2s-1 enabled a
28-fold increase of antibiotic production. Optimization of antibiotic production by
this strain in the continuous culture was undertaken and it was shown that three times
more antibiotic was produced in the continuous culture than in batch culture at the
16th day (Chetsumon et al., 1995).
- Examining the effect of culture conditions on growth and accumulation of
three scytophycin compounds produced by Scytonema ocellatum undertaken by
Patterson & Bolis, 1993 showed that the optimal temperature for production was 250C
and continuous illumination at an intensity of at least 25 µmol photons m-2 s-1 was
required for maximum yield. Growth and metabolites production were optimal in the
pH range of 8.0 to 8.5.
- According to Noaman et al., 2004, an antimicrobial agent is produced by the
cyanobacterium Synechococcus leopoliensis which was found to be active against the
Gram-positive S. aureus. The effects of temperature, pH, incubation period, some
media and different nitrogen and carbon sources on both growth and antimicrobial
activity were investigated. Temperature of 350C and pH 8 were optimum for growth
and antimicrobial agent production. Maximum of growth and antimicrobial activity
were estimated after 14 and 15 days of incubation, in BG-11medium. No
antimicrobial activity could be detected by the use of G medium, moderate activity
was recorded with Chu 10 medium, while high activity was reported in BG-11

Chapter IV Discussion
125
medium. Leucine was the best nitrogen source for antimicrobial activity, while
maximum antimicrobial activity was reached by using the carbon sources, citrate and
acetate. Very high antimicrobial activity was detected by using the carbon source
galactose in combination with the nitrogen source alanine or by using arabinose with
methionine.
- Soltani et al., 2007 showed the effect of salinity (NaCl-free, 0.5 and 1%) on
growth and antimicrobial activity of the cyanobacterium Fischerella sp. FS18.
Aqueous, petroleum ether and methanol extracts of the strain were examined for
activity against 4 bacteria and 2 fungi. The results indicated that the growth rate was
enhanced in NaCl-free medium but the antibacterial activity was higher in medium
with 1% NaCl. It could be explained not only by the role of NaCl in the life cycle of
bacteria but also by decrease in the growth rate of cyanobacteria.
- In studies of Abedin &Taha, 2008, Spirulina platensis was evaluated for
bioactivity against Aspergilus flavus, Fusarium moniliforme, Candida albicans,
Bacillus subtilis, and Pseudomonas aeruginosa by operating the statistical design of
Plackett-Burman for the degree of significance of 8 different trials by using 7
independent variables. The results obtained from Plackett-Burman design revealed
that highest main effect and t-value were detected with NaCl in case of A. flavus while
they were detected with MgSO4 and micronutrients (a) in case of F. moniliforme, with
FeSO4 and micronutrienst (a) with C. albicans. On the other hand, the results revealed
that highest main effects and t-value were detected with micronutrients (b) to B.
subtilis while they were detected with NaCl and K2SO4 in case of P. aeruginosa.
- According to Silva & Silva, 2007, the influence of the mineral nutrients on
the growth of Tolypothrix tenuis was studied and the optimization of mineral nutrients
in culture medium of T. tenius allowed a 73% increase in the final biomass level.
- Optimization studies of biomass production and protein biosynthesis by
Spirulina sp. undertaken by Abu et al., 2007 showed that biomass and protein
produced were significantly (P=0.05) higher under the optimized conditions (pH 9.0;
temperature 300C, 25 mg/L NO3 2-; so on).
- According to Hirata et al., 2003, in a laboratory culture of Noctoc
spongiaeforme, the production of Nostocine A was enhanced at higher temperature
(300C) and more intense light (30 W/m2) than during cultivation under basal
conditions at 250C and 10 W/m2.

Chapter IV Discussion
126
- Imai et al., 2009 reported that in the laboratory experiments where
Microcystic aeruginosa and Microcystic wesenbergii were cultured under various
temperatures, growth rates of M. aeruginosa were significantly higher than those of
M. wesenbergii at high temperatures (30 and 350C) but growth rates of these two
species were similar at lower temperature (20 and 250C).
- The production of phycocyanin and interactions between sodium nitrate,
calcium chloride, trace metal mix and citric acid were investigated and modeled by
Singh et al., 2009. These results showed that under optimized conditions Phormidium
ceylanicum produced a 2.3-fold increased concentration of phycocyanin in
comparison to common used BG-11 medium in 32 days.
In general, optimization of growth media and hence biomass yield will have
profound effects on cell metabolism and subsequently the quantities and types of
secondary compounds synthesized. Secondary metabolite production, considered non-
essential to a cell′s initical survival may not relate directly to high biomass yield;
synthesis of these compounds is often triggered under conditions not conducive to
high growth rates, such as nutrient deprivation (Olaizolá, 2003). Therefore,
optimization of culture conditions should seek to establish a balance between
secondary metabolism and growth rate. As for any microorganisms, the bioprocess
intensification of cyanobacteria is different for every species.
This work is an experiment to optimize the conditions for enhancing the
growth rate and the production of antimicrobial agents by the cyanobacterium
Westiellopsis sp. VN. This strain was selected for culture optimization experiments
because all extracts of Westiellopsis sp.VN possessed the highest strength and widest
range of antimicrobial activity, however, the nutrient factors controlling the
production of these antibiotics by this strain have not been studied so far.
First step was to scale up cultivation from the 500 mL volume in Fehrnbach
flasks to a 35L volume in a fermentor with aeration using air and CO2 to adjust the pH
to 8.5. In contrast to the cyanobacteria Anabaena sp., Nostoc sp., Calothrix
elenkinii, and Scytonema millei which strains did not produce antimicrobial active
metabolites or lower concentrations of these compounds in large scale culture (see
appendix 7), the strain Westiellopsis sp. VN exhibited good growth and production of
antimicrobial active compounds.
Because nitrogen sources fed to the antimicrobial agent producing
cyanobacteria may exhibit significant roles in orienting the secondary metabolic

Chapter IV Discussion
127
pathways (Sailer et al., 1993; Griffiths & Saker, 2003), we have investigated effect of
nitrogen deficiency on biomass production and antibacterial compound accumulation
of this strain. Westiellopsis sp.VN was cultured with BG-11 medium without NaNO3
for 7 weeks under the following culture conditions: temperature 280C, pH 7.4, and
continuous illumination using cool-white fluorescent tubes with an intensity of
8µmol/m2 in a 45 liter-glass fermenter. Cultivation of the strain with the BG-11
standard medium under the culture conditions above-mentioned was used as control.
For evaluation the effects of nitrogen deficiency on yield of lyophilized biomass were
measured. Furthermore the yield of n-hexane, EtOAc, MeOH extracts of biomass and
EtOAc extract of growth medium was estimated and their activity was tested against
S. aureus.
Our studies have shown that the dry biomass weights did not significantly
differ between BG-11 medium without NaNO3 and BG-11 standard medium,
indicating that NaNO3 does not play a role in biomass production of Westiellopsis
sp.VN. In the literature is described for the Nodularia strain GR8b in batch culture
that the highest nitrate concentrations resulted in reduced dry weight (Repka et al.,
2001). Lehtimäki et al., 1994 showed that in laboratory batch cultures, the biomass of
Nodularia spumigena and Aphanizomenon flos-aquae decreased with unnaturally high
inorganic nitrogen concentrations. According to Piorreck et al. 1983, in batch culture,
increasing N level led to an increase of the biomass. This study supports a conclusion
of Soltani et al., 2007 that the good or poor growth of cyanobacteria not only must be
due to their efficiency to metabolize nitrogen but is actually the sum of the entire
physiology and genetics of these organisms.
Concerning the effect of NaNO3 deficiency on synthesis of antimicrobial
substances by this strain in the large scale, cultivation with BG-11 medium without
NaNO3 seems to increase the production of antimicrobial substances in comparison to
BG-11 standard medium. The diameter of inhibition zone of n-hexane and ethyl
acetate extracts from biomass and culture medium respectively cultured in BG-11
without NaNO3 (18.0 mm, 18.0mm, respectively) were bigger than those cultured in
BG-11 standard medium (9.0 mm, 12.5 mm, respectively). Similar results have been
published by Rapala et al., 1997. They have investigated two Anabaena sp. strains, 90
and 202A1 and have shown that the highest levels of microcystins were determined in
a nitrogen-free growth medium. Growth in N-free medium showed that the cells of
Anabaena and Aphanizomenon strains contained more toxin than growth in N-rich

Chapter IV Discussion
128
medium (Rapala et al., 1993). Otherwise decreased toxin production upon removal or
reduction of nitrate from medium was reported for Microcystis aeruginosa (Utkilen
and Giolme, 1995) and for production of nodularin by Nodularia spumigena and
Aphanizomenon flos-aquae at high inorganic nitrogen concentrations (42.000 mg/L)
(Lehtimäki et al., 1997). High toxin production in Oscillatoria agardhii strains also
correlated with high nitrogen concentrations (test range, 0.42 to 84 mg of N per liter)
(Sivonen, 1990). Several species of Microcystis and Oscillatoria synthesize elevated
quantities of toxic secondary metabolites under high nitrogen conditions (Borowitzka,
1999). Thus, different cyanobacteria seem to differ in their responses to external
nitrogen concentrations.
In summary, this study showed that limitation of nitrogen appeared to be
suitable stimulants for accumulation of antimicrobial active substances and nitrogen
concentration had no significant effect on the growth of Westiellopsis sp.VN in the
large scale cultivation. Our results are in general agreement with the results of
Chetsumon et al., 1995 who found that in batch culture immobilized cells of
Scytonema sp. TISTR 8208, secreted the antibiotic after the complete depletion of
nitrate in the medium, but different from results reported by other authors, e.g, Boor
& England, 1991, who reported that increasing nitrate from its base level of 8mM to
26.4mM allowed an increase in antibiotic production of Nostoc muscorum.
4.2.5 Effect of incubation time on biomass production and antimicrobial
compound accumulation of Westiellopsis sp.VN strain
Effect of incubation time on biomass production and antimicrobial compound
accumulation of Westiellopsis sp.VN in batch cultivation with BG 11 medium without
NaNO3 at temperature 20±20C, pH 7.4, and continuous illumination provided by cool-
white fluorescent tube of 8µmol/m2 was investigated. Growth rate of Westiellopsis
sp.VN was monitoring by measure dry biomass weight after 2, 3, 4, 5, 6, 7, and 8
weeks as well as the production of antimicrobial compounds was evaluated by
measuring the diameter of inhibition zone of methanol extracts obtained from dry
biomass in agar diffusion assays using S. aureus. The results showed that the amount
of biomass increased during cultivation time and the biomass yield and diameter of
inhibition zone increased towards the end of the 7-to 8- week growth period but the
inhibition zone of the extract after 8 weeks cultivation is the most clear. From these

Chapter IV Discussion
129
results we conclude that antimicrobial substances were released over the whole
cultivation time by Westiellopsis sp.VN strain. Antimicrobial activity increased fast
at the beginning of the exponential phase (log phase) and continued to increase
gradually over the whole incubation time during log phase to stationary phase.
Antimicrobial activity was the highest after cultivation time of 8- weeks, during the
late stationary phase of the culture.
To date, effect of cultivation age on bioactive compound accumulation of
cyanobacteria was investigated by several groups. Codd et al., 1989 reported that the
cyclic peptide hepatotoxins produced by Microcystis aeruginosa are retained within
the cells during the lag and growth phases, with significant amounts being released
only after the culture becomes senescent and cells begin to lyse. Lehtimäki et al.,
1994 described that in batch cultures, nodularin concentrations in growth media
increased with incubation time indicating release of intracellular nodularin when cells
lysed. In contrast to this Westhuizen & Eloff, 1983 who studied effect of culture age
on toxicity of the blue-green alga Microcystis aeguginosa showed that toxicity
increased gradually during the exponential growth phase to a maximum (LD50 = 18
mg kg -1) at the beginning of the stationary phase and then decreased. Borowitzka,
1995 postulated that synthesis of cyanobacterial metabolites generally occurs during
the late exponential and early stationary phase of organism growth. From our own
results and results of other authors above-mentioned, we conclude that the production
of the active compounds already starts in the exponential growth phase, but the
synthesis enhances clearly during the stationary phase. For isolation of active
compounds in higher yields harvest of the biomass seems to be meaningful in the
stationary phase of the culture.
Influence of physicochemical factors on synthesis of secondary, bioactive
compounds of Westiellopsis sp.VN requires further investigation. The role of other
factors such as pH, temperature, illumination intensity, different N, P, and C sources,
and micronutrients on antimicrobial production should be determined. Furthermore
the influence of competitors in the culture for example heterotrophic bacteria or other
cyanobacteria or microalgae, normally living together with this cyanobacterium in its
natural environment should also be investigated. Future studies are also needed to
determine the genetic or biochemical factors regulating synthesis of antimicrobial
substances by cyanobacteria.

Chapter IV Discussion
130
4.3 Chemical investigation of Calothrix javanica and Scytonema
ocellatum
4.3.1 Selection of Calothrix javanica
In screening 12 different cyanobacterial strains isolated from soil samples in
Dak Lak province of Southern Vietnam for antibacterial activity, we have found that
the extracts of the cyanobacterium Calothrix javanica showed activity against Gram-
positive (B.subtilic and S.aureus) and Gram-negative (E.coli) bacteria. This strain
was therefore selected for bioassay-guided isolation of the active substances.
Morphological identification of the Calothrix javanica isolated from a sample
of rice-field soil collected from EaRoc, Ea Sup, Dak Lak, Vietnam was made in
accordance with traditional phycological system and by using RAPD-PCR technique
(Ho & Vo, 2004; Ho et al., 2006; Ho, 2007).
4.3.2 Selection of Scytonema ocellatum
The extracts obtained from this strain exhibited also strong antimicrobial
activity against Gram-positive bacteria (B. subtilic and S.aureus) in our screening and
this strain was also selected for further chemical investigation. Morphological
identification of the cyanobacterium Scytonema ocellatum isolated from a sample of
rice-field soil collected from EaRoc, Ea Sup, Dak Lak, Vietnam was made in
accordance with traditional phycological system (Ho & Vo, 2004; Vo et al., 2006;
Ho, 2007).
4.3.3 New cyclic peptide of Calothrix javanica and Scytonema ocellatum
According to Houghton & Raman, 1998, extracts from cyanobacteria, leaves
and other green parts of plants made with ethanol, methanol, chloroform and solvents
of similar polarity will contain large amounts of chlorophyll. For removal of
chlorophyll, the reverse-phase column chromatography was used for alcoholic
extracts. This method exploits the non-polar nature of chlorophyll so that it is retained
on a reverse-phase adsorbent while more polar components pass through. With
aforementioned reasons reverse-phase C18 column chromatography was employed
for first purification of the active methanol extract from biomass of Calothrix
javanica. Bioassay-guided fractionation of the active methanol extract prepared from
the lyophilized biomass of the Calothrix javanica strain led to the isolation of a new

Chapter IV Discussion
131
cyclic peptide (3.8mg, 1.89% yield, based on the mass of the crude methanol extract)
exhibiting strong antibiotic activity. The structure of this new compound was
elucidated by exhaustive 1D (1H) and 2D (COSY, TOCSY, NOESY, HMQC,
HMBC) NMR spectroscopy in combination with HR-ESI-MS. The new cyclic
peptide was named as daklakapeptin.
Daklakapeptin was found to have totally 12 residues including 6 proteinogenic
amino acids (Pro, Tyr, Ile, Leu, Gln, Thr), 4 complexes (X,Y,T,Z) and the methyl
derivative of Ile. The exact sequence of daklakapeptin is shown in figure 4-2
Figure 4-2: Sequence
of daklakapeptin
Up to now, there have been only a few scattered reports of the chemistry and
biological activity of the Calothrix genus in the literature. The first mentioned
calophycin, a cyclic decapeptide containing a novel (2R, 3R, 4S)-3-amino-2-hydroxy-
4-methylpalmitic acid unit (Hamp), is a potent broad-spectrum fungicide from
Calothrix fusca EU-10-1 with the MIC against Candida albicans of 1.25µg/ml (Moon
et al., 1992). Then, calothrixins A and B, pentacyclic indolophenanthridines, isolated
from two Calothrix strains, inhibited the growth in vitro of a chloroquine resistant
strain of Plasmodium falciparum, the most virulent strain of plasmodia to humans,
with IC50 values of 58 nM and 180 nM, respectively. These calothrixins also
exhibited potent activity against the human cervical cancer cell line, HeLa, with IC50
values of 40, 350 nM, respectively (Rickards et al., 1999). Calothrixin A also
inhibited RNA synthesis and DNA replication (Doan et al., 2000; 2001). Abarzua et
al., 1999 reported that crude extract of Calothrix brevissima showed very low
antialgal effects against the diatom Nitzschia pusilla. Recently, Berry et al., 2008
reported that five isolates of Calothrix (Calothrix 13-3, Calothrix 30-1-13, Calothrix
3-26, Calothrix 3-27, Calothrix 67-1) from the Florida Everglades and South Florida
contained Calothrixin A. According to Becher et al., 2009, a screening for
cyanobacterial cholinesterase inhibitors had shown that low inhibitory effects on
BChE (butyrylcholinesterase) activity was observed for crude extract of Calothrix
anomala and Calothrix 7507 caused increased activities of BChE compared to the
100% control.
Thr Z Pro Tyr Thr
XIleYTLeuGln
IleNMe

Chapter IV Discussion
132
In present study our new peptide showed antibacterial activity against S
.aureus with the diameter of inhibition zone of 12.5mm in concentration of
200mg/dics (see table 3-13).
Chemical investigation of the active methanol extract resulting from
lyophilized biomass of Scytonema ocellatum led to the isolation of a pure active
compound (2.4mg, 1.21% yield, based on the mass of the crude methanol extract)
which exhibited strong antimicrobial activity with the diameter of inhibition zone of
12 mm in concentration of 200mg/dics (see table 3-20).
Interestingly, the pure active compound from Scytonema ocellatum strain
displayed protonated molecular ion peak at m/z 1432 [M+H]+ in ESIMS positive
mode, the same that was found for the new peptide isolated from the biomass of
Calothrix javanica (see appendix 14c). Moreover, the 1H NMR forms of both
compounds was almost the same (see appendix 14 a,b). In order to verify that the two
compounds isolated from Calothrix javanica and Scytonema ocellatum strains were
identical, the crude methanol extracts prepared from Calothrix javanica and
Scytonema ocellatum strains were analyzed by HPLC with the same conditions. The
results of analytical HPLC revealed that both crude methanol extracts from Calothrix
javanica and Scytonema ocellatum displayed the same peak with tR 15.35 min (see
figure 4-3, 4-4).
Figure 4-3: Analytical HPLC chromatogram of methanol extract of Calothrix javanica with mobile phase of Acetonitrile/ H2O (from 5% to 100% Acetonitrile in 32min) and 2 mg/mL/injection, detection at 246nm, column Synergi POLAR-RP 80A (250×4.6mm, 4 micron), flow rate 1mL/min.

Chapter IV Discussion
133
Figure 4-4: Analytical HPLC chromatogram of methanol extract of Scytonema ocellatum with mobile phase of Acetonitrile/ H2O (from 5% to 100% Acetonitrile in 32min) and 2 mg/mL/injection, detection at 246nm, column Synergi POLAR-RP 80A (250×4.6mm, 4 micron), flow rate 1mL/min.
Thus, it was confirmed that these two compounds isolated by us from
Calothrix javanica and Scytonema ocellatum strains were identical and the same
peptide was synthesized by these two different species. This is interesting but not
surprising because in the literature there are several reports on strains of different
genera within the same family or even on strains of different families can synthesize
identical compounds (Sivonen & Börner, 2008). In our case, Calothrix javanica
belongs to genus Calothrix and Scytonema ocellatum strain belongs to genus
Scytonema, and both of these genera belong to the order Nostocales (Komárek &
Anagnostidis, 1989; 16S rRNA tree showing the close relationships of both genera
shown in fig.4-5).

Chapter IV Discussion
134
Figure 4-5: The phylogenetic relationships of cyanobacteria inferred from 16S rRNA nucleotide sequence (Tomitani et al., 2006)
To date, several strains of the genus Scytonema have been proven to be
producers of bioactive secondary metabolites. Of the genus Scytonema the freshwater
cyanobacterium, Scytonema hofmanni, is known to be highly toxic toward other
cyanobacteria and green algae (Mason et al., 1982). It has been suggested that
Scytonema hofmanni produces allelopathic substances like the chlorine-containing γ-
lactone, cyanobacterin, allowing this slow-growing organism to compete with more
prolific organisms (Pignatello et al., 1983). Besides this, several depsipeptides with
biological activities, especially protease inhibition, have been reported from this
species (Matern et al., 2001; 2003). Additionally, nostodione A previously isolated
from Nostoc commune was also isolated from this strain and nostodione A is known
to be proteasome inhibitor with an IC50 value of 50 µM (Hee et al., 2008).
Scytophycins macrolides that inhibit the proliferation of a wide variety of mammalian
cells were isolated from the genera Cylindrospermum, Scytonema, and Tolypothrix
(Patterson et al., 1994); Scytophycins from Scytonema pseudohofmanni displayed
cytotoxic and antimycotic activity (Ishibashi et al., 1986). Tantazoles and mirabazoles

Chapter IV Discussion
135
are unusual alkaloids from the terrestrial cyanobacterium Scytonema mirable which
show murine and human solid tumour selective cytotoxicity (Carmeli et al., 1990a;
1991, 1993); Scytonemin A, a cyclic peptide, the major metabolite of a Scytonema sp.
strain, possesses potent calcium antagonistic properties on atria at 5µg/mL (diltiazem
was active at 2.5µg/mL) and on portal vein of rat at 20 µg/mL (diltiazem showed
activity at 0.5 µg/mL). Also scytonemin A is weakly active against a wide spectrum
of bacteria and fungi, and is mildly cytotoxic (Helms et al., 1988). A group of known
and new diacylated sulfoglycolipids were isolated from a strain of Scytonema sp.
(Reshef et al., 1997).
Up to now, there have been several reports of the chemistry and biological
activity of the Scytonema ocellatum strain in the literature. Tolytoxin (6-hydroxy-7-
O-methylscytophycin B) and two scytophycins (19-O- demethylscytophycin C, and 6-
hydroxy-7-O-methylscytophycin E) were isolated from this species. These pure
compounds showed MICs of 1-5 ng/ML and 10-50 ng/mL against KB and LoVo,
respectively (Carmeli et al., 1990b). Tolyxin also is a potent antifungal antibiotic,
exhibiting MICs in the range of 0.25 to 8 nM (Patterson & Carmeli, 1992) and
functions as a phytoalexin, a defense agent produced in response to fungal attack
(Patterson & Bolis, 1997). In the present work, new cyclic peptide shows antibacterial
activity against S. aureus.
Both marine and freshwater cyanobacteria have the ability to produce a large
number of peptide metabolites. These cyanopeptide metabolites have enormous
structure diversity ranging from linear to cyclic and multicyclic with simple peptides,
depsipeptides, and lipopeptides structures encompassing a size range of 300-2000 Da.
Many peptides contain nonproteinogenic residues, like D-amino acids, β-amino acids,
hydroxylated and N-methylated amino acids, in addition to proteinogenic amino
acids, which add further to their structure diversity (Van Wagoner et al., 2007).
Several biosynthesis pathways of cyanobacterial peptides have yet to be studied
(Welker & von Döhren, 2006; Van Wagoner et al., 2007, Sivonen et al., 2007;
Gerwick et al., 2008; Jones et al., 2010). These cyanopeptide metabolites possess a
varietyof bioactivity, e.g. antifungal activity (Neuhof et al., 2005), antimalaria activity
(Linington et al., 2007; McPhail et al., 2007), antiviral activity (Zainuddin et al.,
2007), protease inhibition activity (Baumann et al., 2007, Taori et al., 2008),
cytotoxic activity (Linington et al., 2008, Gutiérrez et al., 2008; Tripathi et al., 2009),

Chapter IV Discussion
136
antiprotozoal activity (Simmons et al., 2008), and antimicrobial activity (Zainuddin et
al., 2009).
The cyclic peptides are of particular interest because of their generally high
bioactivity and structural diversity, and the fact that they are phylogenetically very old
(Rantala et al., 2004). The synthesis of cyclic peptides is nonribosomal and controlled
by cassettes of enzymes encoded by gene clusters (Meissner et al., 1996). The gene
clusters are subjected to natural recombination {imperfect repeats, gene loss and
horizontal gene transfer} (Mikalsen et al., 2003), which can explain the large
variation in cyclic peptide sequence. It has been postulated that the gene clusters
coding the nonribosomal peptide synthesis pathways can be engineered to produce
peptides with desired activity such as antibiotic, immunosuppressant or anti-cancer
activity (Neilan et al., 1999). Amino and carboxy termini are themselves linked
together with a peptide bond, forming a circular chain. A number of cyclic peptides
have been discovered in nature and they can range from a few amino acids in length
to hundreds. Cyclic peptides tend to be extremely resistant to digestion, allowing them
to survive intact in the human digestive tract. Several cyclic peptides from
cyanobacteria exhibit antimicrobial activity. For example, kawaguchipeptins A and B
are two cyclic undecapeptides with antibacterial activity isolated from Microcystis
aeruginosa. They inhibit the growth of the Gram-positive bacterium Staphylococcus
aureus at a concentration of 1 µg/mL (Ishida et al., 1997). In our work, new cyclic
peptide showed antibacterial activity against S. aurerus with diameter of inhibition
zone of 12.5 mm in concentration of 200mg/dics. Further tests for activity to other
bacteria and cytotoxic activity are in progress.
4.4 Chemical investigation of Anabaena sp.
4.4.1 Selection of Anabaena sp.
In our antibacterial screening, the crude extracts obtained from Anabaena sp.
strain cultivation medium revealed a strong and wide range of antimicrobial activity
against Gram-positive and Gram-negative bacteria, as well as yeast Candida maltosa.
Biologically active metabolites isolated from cyanobacteria so far have been mostly
accumulated in the cyanobacterial biomass and so far only a couple of bioactive
compounds have been isolated from the cultivation medium e.g., some antibacterial

Chapter IV Discussion
137
diterpenoids from Nostoc commune (Jaki et al., 1999a; 2000a), antifungal peptides
from Tolypotrix byssoidea (Jaki et al., 2001), or the antialgal, antibacterial and
antifungal indole alkaloid norharmane from Nodularia harveyana as well as the 4,4′-
dihydroxybiphenyl from Nostoc insulare (Volk, 2005; Volk & Furkert, 2006). Thus,
this strain was very interesting for bioassay guided isolation of the components in
medium responsible for the antibacterial activity.
Morphological identification of the cynobacterium Anabaena sp. strain
isolated from a sample of industrial cultivating soil (cotton) collected Dak Lak
province of Vietnam was made in accordance with traditional phycological system
(Ho et al., 2005; Ho, 2007).
4.4.2 Active compound of Anabaena sp.
Bioassay-guided isolation of the ethyl acetate extract from the
microscopically cell-free cultivation medium resulted in identification of the
aromadendrane sesquiterpene, flourensadiol, (1.3mg, and 10.83% of the dry extract
weight) which was previously isolated from the common western shrub Flourensia
cernus collected near Big Bend, Texas, USA (Kingston et al., 1975, Pettersen et al.,
1975). This plant is a deciduous shrub endemic to the Chihuahuan Desert of the
southwestern United States and northern Mexico.
The structure of flourensadiol was established using an extensive array of 1D
(1H and 13C, and DEPT-135) and 2D (HMQC, COSY, and HMBC) NMR and HR-
ESI-MS experiments as described in detail in 3.7.
To the best our knowledge, this is the first report on occurrence of an
antibacterial sesquiterpenoid compound in a cyanobacterium and the antibacterial
activity of flourensadiol was also reported for the first time. Kingston et al., 1975 and
Pettersen et al., 1975 determined the structure of the compound by X-ray analysis of
the crystallized compound and published MS, IR, and proton NMR data. In our work,
the structure of flourensadiol was established using an extensive array of 1D (1H , 13C,
DEPT-135) and 2D (HMQC, COSY, HMBC) NMR and HR-ESI-MS experiments,
and the complete NMR data of flourensadiol are reported for the first time.
Occurrence of terpenoids in cyanobacteria is uncommon (Prinsep et al., 1996;
Jaki et al., 1999a, 2000a,b). Some authors published the occurrence of diterpenoids
with antibacterial (Jaki et al., 1999a, 2000b; Gutiérrez et al., 2008), cytotoxic and

Chapter IV Discussion
138
molluscicidal activities (Jaki et al., 2000a), as well as anti-inflammatory activity
(Prinsep et al., 1996). Moreover, further examples are the triterpenoid bacteriophanes
(Simonin et al., 1992) isolated from several species of cyanobacteria. The antifungal
hapalindoles, hapalindolinones, and ambiguines as well as the welwitindolinones are
metabolites of mixed biosynthetic origin containing isoprene units and have been
found in several species of the family Stigonemataceae (Prinsep et al., 1996; Jaki et
al., 1999a). Up to now, there is no information in literature about pharmacological
effects of flourensadiol except a report of Kingston et al., 1975 which indicated that
the plant Flourensia cernua from which flourensadiol was isolated, possess toxicity
to sheep and goat. The toxicity was found in the petrol-insoluble portion which
contained flourensadiol together with other components. In the report of Estell et al.,
1994 it was shown that several of the terpenes identified in tarbush which was
collected in a heavily infested tarbush area on the Jornada Experimental Range
exhibited antimicrobial activity in ruminants. It is of interest to note that in our study
flourensadiol exhibited antibacterial activity against E.coli with diameter of inhibition
zone of 20.0mm in concentration of 200mg/dics but due to the very small amount (1.3
mg) of flourensadiol isolated by our work it was not performed in bioassays for
further activities, but this strain should be investigated for further biological activities.
The natural function of cyanobacterial metabolites so far is unknown but
exometabolites in all likelihood are associated with the interaction between competing
microorganisms of the same habitat (Volk & Furkert, 2006), particularly in resource-
limited environments for example in case of the isolated fluorensiadiol. The common
western shrub Flourensia cernus was collected near Big Bend, Texas, USA and the
Anabaena sp. was isolated from soil of cotton fields of Dak Lak province, both
habitats with poor nutrients and high temperature. It could be most likely that these
sesquiterpenes area involved in defense reactions against cohabitants and improve
chances of survival in their environment.
4.5 Chemical investigation of Nostoc sp.
4.5.1 Selection of Nostoc sp.
The extracts obtained from this strain exhibited strong antimicrobial activity
against Gram-positive bacteria (B. subtilic and S.aureus) and Gram-negative bacteria

Chapter IV Discussion
139
(E.coli) in our screening for cyanobacterial antibacterial activity. Thus, this strain also
was selected for further chemical investigated.
Morphological identification of the cynobacterium Nostoc sp. isolated from
a sample of rice-field soil collected from Dak Lak province, Vietnam was made in
accordance with traditional phycological system (Ho, 2007).
4.5.2 Active compounds of Nostoc sp.
The low resolution ESI-MS of fraction NsF2 which exhibited antibacterial
activity against Staphylococcus aureus with diameter of inhibition zone of 10.0mm in
concentration of 500mg/dics showed signal at m/z 426 [M+H]+. The NMR and MS
characterization of compound in this fraction NsF2 is in progress.
4.6 Chemical investigation of the marine Lyngbya majuscula
4.6.1 Selection of the marine cyanobacterium L. majuscula collected in Vietnam
Marine cyanobacteria would probably ranked along side the actinomycetes
and myxobacteria as a prolific producer of unique natural products. Over the past 30
years, the research for bioactive secondary metabolites (natural products) from marine
organisms has yielded a wealth of new molecules (estimated at approximately 17.000)
with many fundamentally new chemotypes and extraordinary potential for biomedical
research and applications (Blunt et al. 2008). Marine cyanobacteria belong to the most
fruitful sources of marine natural products, with nearly 700 compounds described
(Tan, 2007; Jones et al., 2009).
Majority of the papers is dominated by cyanobacterial collections from reef
systems in Hawaii, the Caribbean, Madagascar, and Papua New Guinea. However, a
few is known on the chemistry of marine cyanobacteria from other parts of the world,
such as South East Asia where biodiversity is high (Tan, 2006). At present, a few is
known on biological activity and chemistry of marine cyanobacteria from Vietnam.
Here is the opportunity to search for novel cyanobacterial biomolecules from these
marine areas which have not been studied intensively so far.
The filamentous marine cyanobacterium Lyngbya majuscula (Gomont) is of
particular importance, as approximately 35% of all cyanobacterial bioactive

Chapter IV Discussion
140
compounds identified so far have been isolated from the genus Lyngbya, with 76% of
these coming from Lyngbya majuscula (Jones et al. 2009). The compounds isolated
from Lyngbya majuscula exhibit a variety of biological activities including
antimicrobial, antiproliferative, immunosuppressant activities. Thus, it is accepted that
the marine cyanobacterium Lyngbya majuscula is an exceptional source of novel
potential pharmaceuticals. Although a lot of studies have been carried out and are still
going on in the research for novel bioactive compounds from Lyngbya majuscula,
there is little report on bioactive compounds isolated from this marine cyanobacterium
growing near the coasts of Vietnam. In our studies, chemical investigation of a
Lyngbya majuscula strain collected in Vietnam at Hon Khoi locality in Khanh Hoa
province was undertaken.
The n-hexane, methanol, and water extracts prepared from lyophilized
biomass of this strain were screened for biological activities including antibacterial,
antifungal and cytotoxic activity. Interestingly, the methanol extract showed strong
cytotoxic activity against 5678 human cell line but weak antimicrobial activity against
Gram-positive bacteria and a yeast (B. subtilis, S. aureus, and C. maltosa with an
inhibition zone of 8, 7, and 9 mm, respectively), and no antibacterial activity against
Gram-negative bacteria (E. coli and P. aeruginosa); therefore this strain was
investigated for compounds responsible for cytotoxic activity.
4.6.2 Cytotoxic compounds of the marine cyanobacterium Lyngbya majuscula
collected in Vietnam
The chemical investigation with an emphasis on the isolation and structure
elucidation of cytotoxic compounds of the methanol extract resulting from this strain
led to the isolation and identification of the 3 cytotoxic compounds
anhydrodebrommoaplysiatoxin (compound 9), debromoaplysiatoxin (compound 10),
and anhydroaplysiatoxin (compound 11). Identification of these cytotoxic compounds
was established by direct comparison of our spectroscopic data, including 1D (1H, 13C) NMR and HR-ESI-MS with those reported in the literature as described in 3.7.1
and 3.7.2.
Debrommoaplysiatoxin (compound 10) was first isolated from the digestive
gland of the sea hare Stylocheilus longicauda (Kato & Scheuer, 1974, 1975) and then
from the marine blue-green alga Lyngbya majuscula (Moore et al., 1984b), but also

Chapter IV Discussion
141
from a mixture of two blue-green algae Schizothrix calcicola and Oscillatoria
nigroviridis (Moore et al., 1984). Compound 10 has been showed to be an activator of
protein kinase C and to be a potent tumor promoter (Fujiki et al., 1981, 1984; Nagai et
al., 1997) and displayed some antineoplastic activity (Mynderse et al., 1977). In our
studies, debrommoaplysiatoxin (compound 10) exhibited cytotoxic activity against
bladder cancer cell line 5637 with IC50 of 86ng/ml.
Anhydrodebromoaplysiatoxin (compound 9) and anhydroaplysiatoxin
(compound 11) were first obtained as artifact during the purification of
debromoaplysiatoxin and aplysiatoxin from the sea hare Stylocheilus longicauda
(Kato & Scheuer, 1976). However, Moore et al., 1984 and Nagai et al., 1998 found
compound 9 as a natural product of L. majuscula collected in Marshall Islands and the
Hawaii Red Alga Gracilaria coronopifolia, respectively. Compound 9 exhibited
antineoplastic activity (Mynderse et al., 1977) and had been reported as potent tumor
promoter (Fujiki et al., 1982; Nagai et al., 1998). In our study, compound 9 and 11
have been identified in the biomass of L. majuscula, the anhydroaplysiatoxin
exhibited cytotoxic activity against bladder cancer cell line 5637 with IC50 of40 ng/ml
but anhydrodebrommoaplysiatoxin was not yet tested for cytotoxic activity.
Notably, Chlipala et al., 2010 reported that nhatrangins A and B,
anhydrodebrommoaplysiatoxin, and anhydroaplysiatoxin have been isolated from a
Vietnamese collection of Lyngbya majuscula. Nhatrangins A and B are described as
two polyketide metabolites in biosynthesis of the aplysiatoxins by this
cyanobacterium. The carbon skeleton of these molecules appears to be related to the
carbon skeleton of the aplysiatoxins and may give an insight into the biosynthesis of
these metabolites. In addition, it is of interest to note that the biomass of the marine
cyanobacterium L.majuscula used by Chlipala et al., 2010 and used in this work were
collected in Nhatrang of Khanh Hoa province, Vietnam, but our sample was collected
in August while the Chlipala sample was collected in May. This may be the reason for
differences in our results comparing with the results of Chlipala et al., 2010, since
differences in environmental conditions in the dry season and the rainy season might
change the production of biologically active metabolites.

Chapter IV Discussion
142
4.7 Conclusion
In our chemical investigation, chemically different and interesting compounds
have been isolated from cyanobacteria. These results promise that further chemical
and biological investigations may provide interesting findings. In further studies,
following points should be included:
- The new cyclic peptide daklakapeptin isolated from Calothrix javanica and
Scytonema ocellatum as well as flourensadiol isolated from Anabaena sp. should
be isolated in sufficient amounts for further biological investigations with a view
to future therapeutic applications.
- The influence of the composition of the cultivation media on the growth rate
and chemical production of cyanobacteria have to be optimized to improve the
synthesis of interesting substances.
- For compounds isolated from the cultivation medium such as flourensadiol, it
would be interesting to study the release mechanisms of these compounds into the
media to optimize the culture conditions and the release rate.
- Because cyanobacteria often produce substances with unusual structure with
bioactive diversity, it is of great important to study the biosynthetic pathways of
these cyanobacteria.
- The hazardous effects, ecological role, and physiological functions of the
cyanobacterial secondary metabolites up to now are poor understood. Thus, these
aspects especially the ecological role of secondary metabolites should be
investigated.
- A comparison of the chemical and morphological characteristics of
cyanobacterial strains which have been cultivated for long time under laboratory
conditions with re-collected organisms from nature should be undertaken in all
likelihood to reveal the environment influences on these organisms.

Summary
143
Summary
Cyanobacteria are a diverse and ancient group of photosynthetic prokaryotic
organisms that can inhabit a wide range of environments including extreme conditions such
as hot springs, desert soils and the Antarctic. They are abundant producers of natural products
well recognized for their bioactivity and utility in drug discovery and biotechnology
applications. Novel intracellular and extracellular compounds from various cultured and field
cyanobacteria with diverse biological activities and a wide range of chemical classes have
considerable potential for development of pharmaceuticals and other biomedical applications.
However, cyanobacteria are still viewed as unexplored source of potential drugs. Especially
the collections of cyanobacterial strains from South East Asia where biodiversity is high are
still largely unexplored. Thus, we investigated twelve soil cyanobacterial strains isolated
from soil samples collected from rice, cotton, and coffee fields in Dak Lak province of
Vietnam and one marine strain, Lyngbya majuscula collected from Khanh Hoa province of
Vietnam for the search for new compounds with antimicrobial and cytotoxic activities.
From the 12 soil cyanobacterial strains, 48 extracts prepared with n-hexane,
methanol, and water for biomasses and ethyl acetate for growth media were screened for
antibacterial activity against Gram-positive bacteria (Bacillus subtilis ATCC 6051 and
Staphylococcus aureus ATCC 6538) and Gram-negative bacteria (Escherichia coli ATCC
11229, Pseudomonas aeruginosa ATCC 27853). Of 48 extracts, 47.92% and 45.83% showed
activity against Bacillus subtilis and Staphylococcus aureus, respectively, while 22.92% and
6.25% exhibited activity against Escherichia coli and Pseudomonas aeruginosa, respectively.
All investigated cyanobacteria (12/12) showed antibacterial activity to at least one of the test
organisms applied. Among the active extracts, extracts obtained from 5 cyanobacterial
strains, Westiellopsis sp. VN, Calothrix javanica, Scytonema ocellatum, Anabaena sp. and
Nostoc sp. showed the highest strength and range of antibacterial activity and therefore were
selected for chemical investigation with an emphasis on the isolation and structure
elucidation of antimicrobial compounds.
Bioassay-guided fractionation of the methanol extract prepared from biomass of
Westiellopsis sp. VN by silica gel chromatography, followed by sephadex LH-20
chromatography and reversed-phase HPLC led to isolation and identification of 6 compounds
as ambiguine D isonitrile, ambiguine B isonitrile, dechloro-ambiguine B isonitrile,

Summary
144
fischerellin A, hydroxy-eicosatetraenoic acid and methoxy-nonadecadienoic acid.
Identification of these active compounds was established by direct comparison of our
spectroscopic data, including 1H NMR and HR-ESI-MS with those reported in the literature.
All these compounds showed biological activity. The identification of fatty acids and other
volatile components by GS-MS in the active MeOH fraction obtained from EtOAc extract of
growth medium was done before commencing further fractionation processes.
Culture optimization of Westiellopsis sp.VN showed that NaNO3 deficiency
increased accumulation of antimicrobial compounds. Biosynthesis of antimicrobial
compounds increased over cultivation time resulting in increased diameter of inhibition zone
of the methanol extract towards the end of the 7-to 8- week growth period, but the most clear
inhibition zone of this extract was detected after cultivation time of 8 weeks.
Bioassay-guided fractionation of the methanol extract prepared from biomass of
either Calothrix javanica by C18 chromatography followed by reversed-phase HPLC or
Scytonema ocellatum by C18 chromatography followed by silica gel chromatography and
reversed-phase HPLC led to isolation and structure elucidation of new cyclic peptide named
daklakapeptin. Structure of daklakapeptin was elucidated by exhaustive 1D (1H) and 2D
(COSY, TOCSY, NOESY, HMQC, HMBC) NMR spectroscopy in combination with HR-
ESI-MS. Daklakapeptin was found to have totally 12 residues including 6 proteinogenic
amino acids (Pro, Tyr, Ile, Leu, Gln, Thr), 4 complexes (X,Y,T,Z) and the methyl derivative
of Ile. The exact sequence of daklakapeptin is shown in following figure
Thr Z Pro Tyr Thr
XIleYTLeuGln
IleNMe
with X: (CH3)2CHCH2CH2CH(NH-)CH2CO-, Y:(CH3)2CHCH(OH)CH(NH-)CO-,
T: HOCH2CH2CH(NH-)CO-, Z: HOCH2CHOHCH(NH-)CO-
This new cyclic peptide exhibited antibacterial activity against Staphylococcus aureus
with diameter of inhibition zone of 12.5 mm in concentration of 200 mg/disc. Further test for
activity to other bacteria and for cytotoxic activity are in progress.
Using reversed-phase HPLC to separate compounds in the crude ethyl acetate extract
obtained from culture medium of Anabaena sp. led to isolation and structure elucidation of
flourensadiol. The structure of flourensadiol was established using an extensive array of 1D
(1H, 13C, DEPT-135) and 2D (HMQC, COSY, HMBC) NMR and HR-ESI-MS experiments.

Summary
145
Flourensadiol was isolated previously from the common western shrub Flourensia cernua.
However, only MS, IR, and proton NMR data but no reports on biological activity were
available. In this study, we report the complete NMR data of flourensadiol for the first time.
Flourensadiol was found to be very strong antibacterial active against Escherichia coli with
diameter of inhibition zone of 20.0 mm in concentration of 200 mg/disc. Further test for
activity to other bacteria and cytotoxic activity are in progress.
Bioassay-guided fractionation of the methanol extract from biomass of Nostoc sp. by
silica gel chromatography followed by C18 chromatography and reversed phase HPLC led to
isolation of the active fraction NsF2 which exhibited antibacterial activity against
Staphylococcus aureus with diameter of inhibition zone of 10.0 mm in concentration of 500
mg/disc. The low resolution ESI-MS of fraction NsF2 showed signal at m/z 426 [M+H]+. The
NMR and MS characterization of compounds in fraction NsF2 is in progress.
Bioassay-guided fractionation of the methanol extract prepared from biomass of
marine cyanobacterium Lyngbya majuscula collected from Khanh Hoa province of Vietnam
by various chromatographic methods (CC, PTLC, HPLC) afforded 3 cytotoxic compounds
anhydrodebromoaplysiatoxin, debromoaplysiatoxin, and anhydroaplysiatoxin. Identification
of these cytotoxic compounds was established by direct comparison of our spectroscopic
data, including (1H, 13C) NMR and HR-ESI-MS with those reported in the literature. In our
study, debromoaplysiatoxin and anhydroaplysiatoxin exhibited cytotoxic activity against
bladder cancer cell line 5637 with IC50 of 86 ng/ml and 40 ng/ml, respectively but
anhydrodebromoaplysiatoxin was not yet tested for cytotoxic activity. The identification of
fatty acids by GS-MS technique in the n-hexane extract obtained from biomass of this marine
cyanobacterium was undertaken before commencing further fractionation processes.
The presented results prove that soil cyanobacteria are a promising source to yield
chemical and pharmaceutical interesting compounds.

146
Rerefences 1. Abarzua, S., Jakubowski, S., Eckert, S. and Fuchs, P. (1999). Biotechnological investigation for
the prevention of marine biofouling II. Blue-green algae as potential producers of biogenic agents for the growth inhibition of microfouling organisms. Botanica Mar. 42, 459–465.
2. Abdel-Raouf, N., and Ibraheem, I. B.M. (2008). Antibiotic activity of two Anabaena species against four fish pathogetic Aeromonas species. Afri. J. Biotechnol. 7, 2644-2648.
3. Abed, R.M., Dobretsov, S., and Sudesh, K. (2009). Applications of cyanobacteria in biotechnology. Journal of applied microbiology 106, 1-12.
4. Abedin, R.M.A., and Taha, H.M. (2008). Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of medium components by Plackett-Burman. Design for antimicrobial activity of Spirulina platensis. Global journal of biotechnology & Biochemistry 3, 22-31.
5. Abu, G. O., Ogbonda, K. H., and Aminigo, R. E. (2007). Optimization studies of biomass production and protein biosynthesis in a Spirulina sp. isolated from an oil-polluted flame pit in the Niger Delta. African, Journal of Biotechnology, 6, 2550-2554.
6. Ame, M.V., Diaz, M., and D.A. Wunderline, D.A. (2003). Occurance of toxic cyanobacterial blooms in San Roque Reservoir (Cordoba, Argentina): a field and chemometric study. Inc. Environ. Toxicol. 18, 192–198.
7. Anagnostidis, K., and Komárek, J. (1990). Modern approach to the classification system of cyanophytes. 5. Stigonematales. Arch. Hydrobiol. Suppl. 80/Algo. Stud. 59, 1-73.
8. Andersen, R.A. (ed) (2005) Algal Culturing Techniques. Elsevier, Amsterdam
9. Asayama, M., Kabasawa, M., Takahashi, I., Aida, T., and Shirai, M. (1996). Highly repetitive sequences and characteristics of genomic DNA in unicellular cyanobacterial strains. FEMS
Microbiol. Lett. 137,175-181.
10. Asthana, R.K., Deepali, Tripathi, M.K., Srivastava, A., Singh, A.P., Singh, S.P., Nath, G., Srivastava, R., and Srivastava, B.S. (2009). Isolation and identification of a new antibacterial entity from the Antarctic cyanobacterium Nostoc CCC 537. J. Appl. Phycol.21, 81-88.
11. Asthana, R.K., Srivastava, A., Singh, A.P., Deepali, Singh, S.P., Nath, G., Srivastava, R., Srivastava, B.S. (2006). Identification of an antimicrobial entity from the cyanobacterium Fischerella sp. isolated from bark of Azadirachta indica (Neem) tree. J. Appl. Phycol. 18, 33-39.
12. Baker, D.D., Chu, M., Oza, U., and Rajgarhia, V. (2007). The value of natural products to future pharmaceutical discovery. Nat.Prod.Rep. 24, 1225-1244.
13. Ballot, A., Dadheech, P.K., and Krienitz, L. (2004). Phylogenetic relationship of Arthrospira, Phormidium, and Spirulina strains from Kenyan and Indian waterbodies. Arch. Hydrobiol. Suppl. 153/Algol. Stud. 113, 37-56.
14. Banker, R., and Carmeli, S. (1998). Tenuecyclamides A-D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var tenue, J. Nat. Prod. 61, 1248–1251.
15. Baracaldo, P.S., Hayes, P.K., and Blank, C.E.(2005). Morphological and habitat evolution in the cyanobacteria using a compartmentalization approach. Geobiology 3,145–165.
16. Barbaras, D., Kaiser, M., Brun,R., and Gademann, K. (2008). Potent and selective antiplasmodial activity of the cyanobacterial alkaloid nostocarboline and its dimmers. Bioorganic & Medicinal
Chemistry Letters 18, 4413-4415.

147
17. Barker, G.L.A., Hayes, P.K., O'Mahony S.L.,Vacharapiyasophon, P., and Anthony E. Walsby, A.E. (1999). A molecular and phenotypic analysis of Nodularia (cyanobacteria) from the Baltic sea. J. Phycol.35, 931-937.
18. Baumann, H.I., Keller, S., Wolter, F.E., Nicholson, G.J., Jung, G., Süssmuth, R., and Jüttner, F. (2007). Planktocyclin, a cyclooctapeptide protease inhibitor produced by the freshwater cyanobacterium Planktothrix rubescens. J. Nat. Prod. 70, 1611-1615.
19. Becher, P.G., Baumann, H.I., Gademann, K., and Juettner, F. (2009). The cyanobacterial alkanoid nostocarboline: an inhibitor of acetylcholinesterase and trysin. J. Appl. Phycol. 21, 103-110.
20. Becher, P.G., Keller, S., Jung, G., Suessmuth, R.D., and Juetter, F. (2007). Insecticidal activity of 12- epi-hapalindole J isonitrile. Phytochemistry 68, 2493-2497.
21. Beck, Z. Q., Aldrich, C. C., Magarvey, N. A., Georg, G. I. and Sherman, D. H. (2005). Chemoenzymatic synthesis of cryptophycin/arenastatin natural products. Biochemistry 44, 13457– 13466.
22. Beckwith M, Urba WJ, and Longo DL. (1993). Growth inhibition of human lymphoma cell lines by the marine products, dolastatins 10 and 15. J. Natl. Cancer Inst, 85, 483–8.
23. Ben-Porath, J., and Zehr, J. P. (1994). Detection and characterization of cyanobacterial nifH genes. Appl. Environ. Microbiol. 60, 880-887.
24. Berry, J.P., Gantar, M., Gawley, R.E., Wang, M.L., and Rein, K.S. Pharmacology and toxicology of pahayokolide A, a bioactive metabolite from a freshwater species of Lyngbya isolated from the Florida Everglades. Comp Biochem. Phys. C Toxicol Pharmacol. 139, 231-238.
25. Berry, J.P., Gantar, M., Perez, M.H., Berry, G., and Noriega, F.G. (2008). Cyanobacterial toxins as allelochemicals with potential applications as algaecides, herbicides and insecticides. Mar.
Drugs 6, 117–146.
26. Bewley CA, Gustafson KR, Boyd MR, Covell DG, Bax, Clore GM, Gronenborn AM (1998). Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat Biol 5, 571-578
27. Bhadury, P., and Wright, P.C. (2004). Exploitation of marine algae: biogenic compounds for potential antifouling applications. Planta 219, 561-578.
28. Biondi, N., Piccardi, R., Margheri, M.C., Rodolfi, L.,Smith, G.D., and Tredici, M.R. (2004). Evaluation of Nostoc strain ATCC 53789 as a potential source of natural pesticides. Appl. Environ. Microbiol 70, 3313–3320.
29. Blom, J.F., Brütsch, T., Barbaras, D., Bethuel,Y., Locher, H.H., and Hubschwerlen, C. (2006). Potent algicides based on the cyanobacterial alkaloid nostocarboline. Org. Lett. 8, 737–740.
30. Bloor, S., and England, R.R. (1991). Elucidation and optimization of the medium constituents controlling antibiotic production by the cyanobacterium Nostoc muscorum. Enzyme and Microbial Technology 13, 76-81.
31. Blunt, J.W., Copp, B.R., Hu, W.P., Munro, M.H.G., Northcote, P.T., and Prinsep, M. R. (2008). Marine natural products. Nat. Prod. Rep. 25, 35-94.
32. Blunt, J.W., Copp, B.R., Hu, W.P., Munro, M.H.G., Northcote, P.T., and Prinsep, M. R. (2010). Marine natural products. Nat. Prod. Rep. 27, 165-237.
33. Bokesch, H., O'Keefe, B.R., Tawnya C. McKee, Lewis K. Pannell,Gregory M. L. Patterson, Roberta S. Gardella, Raymond C. Sowder, II,Jim Turpin,Karen Watson, Robert W. Buckheit, Jr., and Michael R. Boyd (2003). A Potent Novel Anti-HIV Protein from the Cultured Cyanobacterium Scytonema varium. Biochemistry 42, 2578–2584

148
34. Bolch, C.J.S., Blackburn, S.L., Neilan, B.A., and Grewe, P.M. (1996). Genetic characterization of strains of cyanobacteria using PCR-RFLP of the cpcBA intergenic spacer and flanking regions. J. Phycol. 32, 445-451
35. Bonjouklian, R., Tim A. Smitka, Larry E. Doolin, R. Michael Molloy, Manuel Debono and Stacey A. Shaffer, Richard E. Moore*, Jeffrey B. Stewart and Gregory M. L. Patterson. (1991). Tjipanazoles, new antifungal agents from the blue-green alga Tolypothrix tjipanasensis. Tetrahedron 47, 7739-7750.
36. Borowitzka M.A. (1999). Pharmaceuticals and agrochemicals from microalgae. In. Chemicals from microalgae. Edited by Z.Cohen, Taylor&Francis Ltd. 313-352.
37. Borowitzka, M.A (1995). Microalgae as sources of pharmaceuticals and other biologically active compounds. J.Appl. Phycol.7, 3–15.
38. Boyd, M.R., K R Gustafson, J B McMahon, R H Shoemaker, B R O'Keefe, T Mori, R J Gulakowski, L Wu, M I Rivera, C M Laurencot, M J Currens, J H Cardellina, 2nd, R W Buckheit, Jr, P L Nara, L K Pannell, R C Sowder, 2nd, and L E Henderson. (1997). Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother 41, 1521–1530.
39. Breier, A., Barancik, M., Sulova, Z. and Uhrik, B. (2005). P-Glycoprotein–implications of metabolism of neoplastic cells and cancer therapy. Curr. Cancer Drug Targets 5, 457– 468.
40. Bui, H.T., Jansen, R., Pham, H.T., Mundt, S. (2007). Carbamidocyclophanes A-E, chlorinated paracyclophanes with cytotoxic and antibiotic activity from the Vietnamese cyanobacterium Nostoc sp. J. Nat. Prod. 70, 499-503.
41. Burja, A.M., Banaigs, B., Abou-Mansour, E., Burgess, J.G. and Wright, P.C. (2001). Marine cyanobacteria – a prolific source of natural products. Tetrahedron 57, 9347- 9377.
42. Butler, M. S. (2008). Natural products to drugs: natural product-derived compounds in clinical trials. Natural Product Reports, 25, 457-516.
43. Cannell, R. J.P. (ed) (1998). Natural products isolation. Humanna Press, Totowa, New Jersey.
44. Carmeli, S., Moore, R.E., Patterson, G.M.L. (1990b). Tolytoxin and new scytophycins from three species of Scytonema. J. Nat. Prod.53, 1533-1542
45. Carmeli, S., Moore, R.E., Patterson, G.M.L. (1991). Mirabazoles, minor tantazole-related cytotoxins from the terrestrial blue-green alga Scytonema mirabile. Tetrahedron Lett. 32, 2593-2596.
46. Carmeli, S., Moore, R.E., Patterson, G.M.L., Corbett, T.H., and Valeriote, F.A. (1990a). Tantazoles: unusual cytotoxic alkaloids from the blue-green algal Scytonema mirabile. J.Am.Chem. Soc. 112, 8195-8197.
47. Carmeli, S., Paik, S., Moore, R.E., Patterson, G.M.L., Yoshida, W.Y. (1993). Revised structures and biosynthetic studies of tantazoles A and B. Tetrahedron Lett. 34, 6681-6684.
48. Carter, D. C., R. E. Moore, J. S. Mynderse, W. P. Niemczura, J. S. Todd. (1984). Structure of majusculamide C, a cyclic depsipeptide from Lyngbya majuscula. J. Org. Chem. 49, 236-41.
49. Castenholz, R.W. (1988). Culturing methods for cyanobacteria. In: Methods in enzymology Vol.167 (Packer L. Glazer AN, eds.), Academic Press, San Diego, 68-93.
50. Castenholz, R.W. (2001) Cyanobacteria et al. In : Boone, D.R., Castenholz, R.W., and Garrity, G.M. (Eds.) Bergey’s Manual of Systematic Bacteriology, 2nd Ed., The Archaea and the Deeply Branching and Phototrophic Bacteria. Springer -Verlag, NY. 1, 473-597

149
51. Chen, J., and Forsyth, C.J. (2004). Total synthesis of the marine cyanobacterial cyclodepsipeptide apratoxin A. Proc. Natl. Acad. Sci. U.S.A. 101, 12067-12072.
52. Chetsumon, A., Fujieda, K., Hirata, K., Yagi, K., and Miura, Y. (1993). Optimization of antibiotic production by the cyanobacteriumScytonema sp. TISTR 8208 immobilized on polyurethane foam . J. Appl. Phycol. 5, 615-622.
53. Chetsumon, A., Maeda, I., Umeda, F., Yagi, K., and Miura, Y., and Mizoguchi, T. (1995). Continuous antibiotic production by an immobilized cyanobacterium in a seaweed-type bioreactor. J.Appl.Phycol. 7, 135-139.
54. Chetsumon, A., Miyamoto, K., Hirata, K., Miura, Y., Ikuta, Y., and Hamsaki, A. (1993). Factors affecting antibiotic production in bioreactors with immobilized algal cells. Appl. Biochem. Biotech. 37, 573-586.
55. Chlipala, G., Shunyan Mo, Esperanza J. Carcache de Blanco, Aiko Ito, Stanley Bazarek, Jimmy Orjala. (2009). Investigation of antimicrobial and protease-inhibitory activity from cultured cyanobacteria. Pharmaceutical Biology 47, 53-60.
56. Chlipala, G., Tri, P.H, Hung, N.V., Krunic, A., Shim, S.H., Soejarto, D.D., and Orjiala. (2010). Nhatrangins A and B, aplysiatoxin-related metabolites from the marine cyanobacterium Lyngbya majuscula from Vietnam. J. Nat. Prod. 73, 784-787
57. Clardy, J., and Walsh, C. (2004). Lessons from natural molecules. Nature, 432, 829-837
58. Codd, G.A., Bell, S.G., Kaya, K., Ward, C.J., Beattie, K.A., and Metcalf, J.S. (1999). Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 34, 405–415.
59. Cold, G.A., Morrison, L. F. M., Metcalf, J. S. (2005). Cyanobacterial toxins: risk management for health protection. Toxicol. Appl. Pharm. 203, 264-272.
60. Cole, M.D. (1994). Key antifungal, antibacterial and anti-insect assays- a critical review. Biochem. Syst. Ecol. 22, 837-856.
61. Corbett, T. H., Valeriote, F. A., Demchik, L., Lowichik, N., Polin, L., Panchapor, C., Pugh, S., White, K., Kushner, J., Rake, J., Wentland, M., Golakoti, T., Heltzel, C., Ogino, J., Patterson, G. and Moore, R. (1997). Discovery of cryptophycin-1 and BCN-183577: examples of strategies and problems in the detection of antitumor activity in mice. Invest. New Drugs 15, 207– 218.
62. D'Agostino, G., del Campo, J., Mellado, B., Izquierdo, M. A., Minarik, T., Cirri, L., Marini, L., Perez-Gracia, J. L. and Scambia, G. (2006) A multicenter phase II study of the cryptophycin analog LY355703 in patients with platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer 16, 71– 76.
63. Dahms, H.-U., Xu, Y. and Pfeiffer, C. (2006). Antifouling potential of cyanobacteria: a mini-review. Biofouling 22, 317–327.
64. Davidson, B. S. (1995). New dimensions in natural products research: cultured marine microorganisms. Curr.Opin.Biotechnol 6, 284-291.
65. De Caire G.Z., De Cano M.M.S., De Mule M.C.Z., and De Halperin D.R. (1993). Screening of cyanobacterial bioactive compounds against human pathogens. Phyton. 54, 59-65.
66. De Cano, M. M. S., de Mulé, M. C. Z., de Caire, G. Z., and de Halperin, D. R. (1990). Inhibition of Candida albicans and Staphylococcus aureus by phenolic compounds from the terrestrial cyanobacterium Nostoc muscorum. J. Appl. Phycol. 2, 79-81.
67. de Jonge, M.J., van der Gaast, A., Planting, A.S., van Doorn, L., Lems, A., Boot, I., Wanders, J., Satomi, M., Verweij, J. (2005). Phase I and pharmacokinetic study of the dolastatin 10 analogue TZT-1027, given on days 1 and 8 of a 3-week cycle in patients with advanced solid tumors. Clin Cancer Res 11, 3806–3813.
68. De Philippis R., and Vincenzini. M. (1998). Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev. 22, 151–175

150
69. Dismukes, G. C., Klimov, V.V., Baranov, S.V., Kozlov, Y.N., DasGupta, J., and Tyryshkin, A. (2001). The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc. Nat. Acad. Sci. USA. 98, 2170-2175.
70. Dixon, R.A., Al-Zazawi, M., and Alderson,G. (2004). Permeabilising effects of sub-inhibitory concentrations of microcystin on the growth of Escherichia coli. FEMS Microbiol Lett. 230, 167–170
71. Doan, N.T., Rickards, R.W., Rothschild, J.M., Smith, G.D. (2000). Allelopathic actions of the alkaloids 12-epi-hapalindole E isonotrile and calothrixin A from cyanobacteria of the genera Fischerella and Calothrix. J. Appl. Phycol.12, 409-416.
72. Doan, N.T., Stewart, P.R., Smith, G.D. (2001). Inhibition of bacterial RNA polymerase by the cyanobacterial metabolites 12-epi-hapalindole E isonotrile and calothrixin A. FEMS Microbiology Letters 196, 135-139.
73. Dunlap, W.C., Battershill. C.N., Liptrot, C.H., Cobb, R.E., Bourne, D.G., Jaspars, M., Long, P.F., and Newman, D.J. (2007) Biomedicinals from the phytosymbionts of marine invertebrates: A molecular approach. Methods 42, 358-376.
74. Dyble, J., Paerl, H.W., and Neilan, B.A. (2002). Genetic characterization of Cylindrospermopsis raciborskii (cyanobacteria) isolates from diverse geographic origins based on nifH and cpcBA-IGS nucleotide analysis. Appl. Environ. Microbiol. 68, 2567-2571.
75. Edelman, M. J., Gandara, D. R., Hausner, P., Israel, V., Thornton, D., DeSanto, J. and Doyle, L. A. (2003). Phase 2 study of cryptophycin 52 (LY355703) in patients previously treated with platinum based chemotherapy for advanced non-small cell lung cancer. Lung Cancer 39, 197– 199.
76. Eggen, M.J. and Georg, G.I. (2002). The cryptophycins: their synthesis and anticancer activity. Med. Res. Rev. 22, 85-101.
77. Egorov, N.S. (1985). Antibiotics a Scientific Approach. Mir Publishers, Moscow
78. Entzeroth M., Blackman, A. J., Mynderse, J.S., Moore, R.E. (1985). Structures and stereochemistries of oscillatoxin B, 31-noroscillatoxin B, oscillatoxin D, and 30-methyloscillatoxin D. J.Org.Chem. 50, 1255–1259.
79. Estell, R.E., Havstad, K.M., Fredrickson, E.L., and Gardea-Torresdey, J.L. (1994). Secondary chemistry of the leaf surface of Flourensia cernua. Biochemical systematics and Ecology 22, 73-77.
80. Falch, B.S., Koenig, G.M., Wright, A.D., Sticher, O., Rueegger, H. and Bernardinelli, G. (1993). Ambigol A and B—new biologically active polychlorinated aromatic compounds from the terrestrial blue-green alga Fischerella ambigua. J.Org. Chem. 58, 6570–6575.
81. Falch, B.S., Konig, G.M., Wright, A.D. (1955). Biological activities of cyanobacteria: evaluation of extracts and pure compounds. Plant. Med. 61, 321-328.
82. Fergusson, K.M., and Saint, C.P. (2000). Molecular phylogengy of Anabaena circinalis and its identification in environment samples by PCR. Appl.Environ. Microbiol. 66, 4145-4148.
83. Fischetti L, Barry SM, Hope TJ, Shattock RJ (2009). HIV-1 infection of human penile explants tissue and protection by candidate microbicides. Aids 23, 319-328
84. Flores, E., and Wolk, C.P. (1986). Production, by filamentous, nitrogen-fixing cyanobacteria, of a bacteriocin and of other antibiotics that kill related strains. Arch. Microbiol. 145, 215-219.
85. Fojo, A. T., and Menefee, M. (2005) Microtubule targeting agents: basic mechanisms of multidrug resistance (MDR). Semin. Oncol. 32, S3– S8.
86. Frassanito, R., Cantonati, M., Tardio, M., Mancini, I., and Guella, G. (2005). On-line identification of secondary metabolites in freshwater microalgae and cyanobacteria by combined

151
liquid chromatography-photodiode array detection-mass spectrometric techniques. J.
Chromatography A 1082, 33-42.
87. Fujiki, H., Mori, M., Nakayasu, M., Terada, M., Sugimura, T, and Moore, R. E. (1981). Indole alkaloids: dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters. Proc. Natl. Acad. Sci. USA. 78, 3872-3876.
88. Fujiki, H., Tanaka, Y.,Miyake, R., Kikkawa, U., Nishizuka, Y., Sugimura, T. (1984). Activation of calcium-activated, phospholipid-dependent protein kinase (protein kinase C) by new classes of tumor promoters: Teleocidin and debromoaplysiatoxin. Biochemical and Biophysical Research Communications 120, 339-343.
89. Fujiki, H; Suganuma, M; Nakayasu, M; Hoshino, H; Moore, R.E and Sugimura, T. (1982). The third class of new tumor promoters, polyacetates (debromoaplysiatoxin and aplysiatoxin), can differentiate biological actions relevant to tumor promoters. Gann, 73, 495-497.
90. Gademann, K., and Kobylinska, J.(2009). Antimalarial natural products of marine and freshwater origin. The Chemical Record. 9, 187-198.
91. Gademann, K., and Portmann, C. (2008). Secondary metabolites from cyanobacteria: complex structures and powerful bioactivities. Curr. Org. Chem. 12, 326–341.
92. Gailliot, F.P. (1998). Initial extraction and product capture. In: Natural products isolation (Cannell RJP, ed.), Humana Press, Totowa, New Jersey, 53-89.
93. Gantar, M., Berry, J.P.,Thomas, S., Wang, M.,Perez, R.,Rein, K.S., and King, G. (2008). Allelopathic activity among cyanobacteria and microalgae isolated from Florida freshwater habitats. FEMS Microbiol. Ecol. 64, 55–64.
94. Gerwick, W. H., Proteau, P.J.,Nagle, D.G.,Hamel, E., Blokhin, and A.,Slate, D.L. (1994b). Structure of Curacin A, a Novel Antimitotic, Antiproliferative and Brine Shrimp Toxic Natural Product from the Marine Cyanobacterium Lyngbya majuscula. J. Org.Chem. 59, 1243-1245.
95. Gerwick, W. H., Reyes, S., and Alvarado, B. (1987). Two malyngamides from the Carribean cyanobacterium Lyngbya majuscula. Phytochemistry 26, 1701-4.
96. Gerwick, W., Byrum, T., Carland, T., Coates, R.C., Engene, N., Esquenazi, E., Gerwick, L., Grindberg, R., Johnson, M., Jones, A., Malloy, K., Nunnery, J., Pereira, A., Soria, I., Sorrels, C., Taniguchi, M., Tidgewell, K., Villa, F., Vining, O., Dorrestein, P., Gu, L., Sherman, D.H. (2008). Integrating chemical and biochemical approaches to natural products drug discovery from marine cyanobacteria. In International conference drug discovery from natural products and traditional medicines (DDNPTM-08), 33-43.
97. Gerwick, W.H., Byrum, T., Carland, T., Coates, R. C., Engene, E.E., Gerwick, L., Grindberg, R., Johnson, M., Jones, A., Malloy, K., Nunnery, J., Pereira, A., Soria, I., Sorrel, C., Taniguchi, M., Tidgewell, K., Villa, F., Vining, O., Dorrestein, P., Gu, L., and Sherman, D. (2008). Integrating chemical and biochemical approaches to natural products drug discovery from marine cyanobacteria. International Conference on Newer Developments in Drug Discovery from Natural Products and Traditional Medicines (DDNPTM-08), 33-43.
98. Gerwick, W.H., Roberts, M.A., Proteau, P.J., and Chen, J.L. (1994a). Screening cultured marine microalgae for anticancer-type activity. J.Appl.Phycol. 6, 143-149.
99. Gerwick, W.H., Tan, L.T., and Sitachitta, N. (2001). Nitrogen-containing metabolites from marine cyanobacteria. In: Cordell, G.A.(Ed.), . In: The Alkaloids: Chemistry and Biology. Acedemic Press, San Diego 57, 75-184.
100. Ghasemi, Y., Yazdi, M.T., Shafiee, A., Amini, M., Shokravi, S., Zarrini G., (2004). Parsiguine, a novel antibacterial substance from Fischerella ambigua. Pharmaceut. Biol. 42, 318-322.

152
101. Giovannoni, S.J., Turner, S., Olsen, G.J., Barns, S., Lane, D.J., Pace, N.R.(1988). Evolutionary relationships among cyanobacteria and green chloroplasts. J. Bacteriol. 170, 3584-3592.
102. Gleason, F.K., and Case, D.E. (1986). Activity of the natural algicide, cyanobacterin, on angiosperms. Plant Physiol. 80, 834–837.
103. Greuter, W., McNeill, J., Barrie, F.R., Burdet, H.M., Demoulin, V., Filgueiras, T.S., Nicolson, D.H., Silva, P.C., Skog, J.E., Trehane, P., Turland, N.J., and Hawksworth, D.L. (2000). International code of botanical nomenclature (St Louis code). Regnum Vegetabile 138. Koeltz Scientific Books. Königstein.
104. Greystoke, A., Blagden, S., Thomas, A.L., Scott, E., Attard, G., Molife, R., Vidal, L., Pacey, S., Sarkar, D., Jenner, A., De-Bono, J.S., and Steward, W. (2006). A phase I study of intravenous TZT-1027 administered on day 1 and day 8 of a three-weekly cycle in combination with carboplatin given on day 1 alone in patients with advanced solid tumours. Ann. Oncol. 17, 1313–1319.
105. Griffiths, D.J., and Saker, M.L. (2003). The Palm island mystery disease 20 years on: a review of research on cyanotoxin cylindrospermopsin, Inc. Environ. Toxicol. 18, 78–93
106. Gromov, B.V., Vepritskiy, A.A., Titova, N.N., Mamkayeva, K.A., and Alexandrova, O.V. (1991). Production of the antibiotic cyanobacterin LU-1 by Nostoc linckia Calv 892 (Cyanobacterium), J. Appl. Phycol. 3, 55–59.
107. Gross, E.M. (2003). Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22, 313-339.
108. Gugger, M., Lyra, C., Henriksen, P., Coute, A., Humbert, J.F., and Sivonen, K., (2002). Phylogenetic comparison of the cyanobacterial genera Anabaena and Aphanizomenon. Int J Syst Evol Microbiol 52,1867-1880.
109. Gustafson, K.R., Cardellina, J.H., Fuller, R.W., Weislow, O.S., Kiser, R.F., Snader, K.M., Patterson, G.M.L., and M.R. Boyd, M.R. (1989). AIDS-antiviral sulfolipids from cyanobacteria (blue-green algae). J. Natl. Cancer. Inst. 81, 1254–1258.
110. Gutiérrez, M., Suyama, T.K., Engene, N., Wingerd, J. S., Matainaho, T., and Gerwick, W.H. (2008). Apratoxin D, a potent cytotoxic cyclodepsipeptide from Papua new Guinea collections of the marine cyanobacteria Lyngbya majuscule and Lyngbya sordida. J. Nat. Prod. 71, 1099-1103.
111. Hagmann, L., and Jüttner, F. (1996). Fischerellin A, a novel photosystem-II-inhibiting allelochemical of the cyanobacterium Fischerella muscicola with antifungal and herbicidal activity. Tetrahedron Letters 37, 6425-6622.
112. Hamburger, M.O., and Cordell, G.A. (1987). A direct bioautography TLC assay for compounds possessing antibacterial activity. J. Nat. Prod. 50,19-22.
113. Hamel, E., Covell, D.G. (2002). Antimitotic peptides and depsipeptides. Curr Med Chem
Anti-Canc Agents 2, 19–53.
114. Harvey, A. (2000). Strategies for discovering drugs from previously unexplored natural products. Drug Discov Today 5, 294-300.
115. Harvey, A.L. (2008). Natural products in drug discovery. Drug discovery today. 13, 894-901.
116. Hayashi, K., Hayashi,T., Morita, N., and Kojima, I. (1993). An extract from Spirulina platensis is a selective inhibitor of Herpes simplex virus type-1 penetration into HeLa cells. Phytotherapy Research 7, 76–80.
117. Hayashi,T., Hayashi, K., Maeda, M. and Kojima, I. (1996). Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 59, 83–87.

153
118. Hedges, S.B., Chen, H., Kumar, S., Wang, D.Y.-C., Thompson, A. S., and Watanabe, H. (2001). A genomic timescale for the origin of eukaryotes. BMC Evol. Biol. 1: 4.
119. Hee, S.S., Chlipala, G., and Orjala, J. (2008). Isolation and Structure Determination of a Proteasome Inhibitory Metabolite from a Culture of Scytonema hofmanni. J. Microbiol.
Biotechnol. 18, 1655-1658.
120. Helms, G.L., Moore, R.E., Niemczura, W.P., Patterson, G.M.L. (1988). Scytonemin A, a novel calcium antagonist from a blue-green alga. J.Org. Chem. 53, 1298-1307.
121. Hirata, K., Yoshitomi, S., Dwi, S., Iwabe, O., Mahakhant, A., and J. Polchai, J., and Miyamoto, K. (2003). Bioactivities of nostocine A produced by a freshwater cyanobacterium Nostoc spongiaforme TISTR 8169. J. Biosci. Bioeng. 95, 512–517.
122. Ho, S. H. (2007). "Cyanobacria in some soil areas of Dak Lak province and the relationship between them with some ecological factors". Ph.D thesis
123. Ho, S. H., Vo, H. (2004) Cyanobacteria in the ricefields of Daklak province. Proceedings, the 2004th National conference on life sciences Thai Nguyen University, Science and Technics publishing House November 23, 88-91.
124. Ho, S. H., Vo, H., and Duong, D.T. (2005b). Cyanobacteria in the industrial cultivating soils (cotton and coffee) of Dak Lak province. Proceedings, the 2005th National conference on life sciences Hanoi Medical University, Science and Technics publishing House November 03, 2005, 920-923.
125. Ho, S.H., Vo, H., Dang, D.H., Duong, D.T. (2005a), New finding of genus Westiellopsis Janet from rice-field soil of the Dak Lak province for Vietnamese microalgae. J. Biotechnol. 3 (4): 509-516.
126. Ho, S.H., Vo, H., Duong, D. Tien, Dang Diem Hong (2006), Using RAPD-PCR technique to identify the genetic relationship of genus Calothrix species isolated from cultural soil soil of the DacLac province. J. Biol. 28(1): 75-80.
127. Hoffmann, L., Komárek J., and Kaštovský, J. (2005). System of cyanoprokaryotes (cyanobacteria)-state in 2004. Arch. Hydrobiol. Suppl. 159/Algol. Stud. 117, 95-115.
128. Houghton, P. J., and Raman, A. (1998). Laboratory Handbook for the Fractionation of Natural Extracts. Published in Chapman & Hall, an imprint of Thomson Science, 2-6 Boundary Row, London SE1 8HN, UK
129. Huber, U., Moore, R.E., and Patterson, G.M.L. (1998). Isolation of a Nitrile- containing Indole Alkaloid from the Terrestrial blue-green alga Hapalosiphon delicatulus. J. Nat. Prod. 61, 1304-1306.
130. Huskens D, Vermeire K, Profy AT, Schols D (2009). The candidate sulfonated microbicide, PRO 2000, has multiple mechanisms of action against HIV-1. Antiviral Res 84, 38-47
131. Imai, H., Chang, K., Kusaba, M., and Nakkano, S. (2009). Temperature-dependent dominance of Microcystis (cyanophyceae) species: M. aeruginisa and M. wesenberbii. Journal of Plankton research, 31, 171-178.
132. Ishibashi, F., S. Park, T. Kusano and K. Kuwano. (2005). Synthesis and algicidal activity of (+)-cyanobacterin and its stereoisomer. Biosci. Biotechnol. Biochem. 69, 331–396.
133. Ishibashi, M., Moore, R.E., Patterson, G.M.H., Xu, C., and Clardy, J. (1986). Scytophycins, cytotoxic and antimycotic agents from the cyanophyte Scytonema pseudohofmanni. J.Org. Chem. 51, 5300-5306.
134. Ishida, K., Matsuda, H., Murakami, M., and Yamaguchi, K. (1997). Kawaguchipeptin A, an antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 60, 724-726.

154
135. Issa, A.A. (1999). Antibiotic production by the cyanobacteria Oscillatoria angustissima and Calothrix parietina. Environ. Toxicol. Pharm. 8, 33–37.
136. Itou, Y.; Ishida, K.; Shin, H. J.; Murakami, M. (1999). Oscillapeptins A to F, serine protease inhibitors from the three strains of Oscillatoria agardhii. Tetrahedron, 55, 6871-6882.
137. Jaiswal, P., Singh, P.K., and Prasanna,R. (2008). Cyanobacterial bioactive molecules—an overview of their toxic properties. Can. J. Microbiol. 54, 701-717.
138. Jaki, B., Heilmann, J., and Sticher, O. (2000b). New antibacterial metabolites from the cyanobacterium Nostoc commune (EAWAG 122b). J. Nat. Prod. 63, 1283–1285.
139. Jaki, B., Orjala, J., Bürgi, H.R., Sticher, O. (1999b). Biological screening of cyanobacteria for antimicrobial and molluscicidal activity, brine shrimp lethality, and cytotoxicity. Pharmaceut. Biol. 37, 138–143.
140. Jaki, B., Orjala, J., Heilmann, J. Linden, A., Vogler, B., and Sticher, O. (2000a). Novel extracellular diterpenoids with biological activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 63, 339-343.
141. Jaki, B., Orjala, J., Sticher, O. (1999a). A novel extracellular diterpenoid with antibacterial activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 62, 502-3.
142. Jaspars, M., and Lawton, L. (1998). Cyanobacteria- a novel source of pharmaceuticals. Current Opinion in Drug Discovery and Development 1, 77-84.
143. Jones, A.C., Gerwick, L., Gonzalez, D., Dorrestein, P.C., William H Gerwick, W.H. (2009). Transcriptional analysis of the jamaicamide gene cluster from the marine cyanobacterium Lyngbya majuscula and identification of possible regularory proteins. BMC Microbiology 9, 247.
144. Jones, A.C., Gu, L., Sorrels, C.M., Sherman, D.H., and Gerwick, W.H. (2009). New tricks from ancient algae: natural products biosynthesis in marine cyanobacteria. Current Opinion in Chemical Biology 13, 216-223.
145. Jones, A.C., Monroe, E.A., Eisman, E.B., Gerwick, L., Sherman, D.H., and Gerwick, W.H. (2010). The unique mechanistic transformations involved in the biosynthesis of modular natural products from marine cyanobacteria. Nat.Prod. Rep.27, 1048
146. Jordan, M.A., Wilson, L. (1998). Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 10, 123-130.
147. Junichi, W., Tsugitaka, N., and Motohiro, K. (2007). Comparison of the antivascular and cytotoxic activities of TZT-1027 (Soblidotin) with those of other anticancer agents. Anti-Cancer Drugs. 18, 905-911.
148. Kalaitzis, J. A., Lauro, F.M., and Neilan B. A. (2009). Mining cyanobacterial genomes for genes encoding complex biosynthetic pathways. Nat. Prod. Rep. 26, 1447-1465
149. Kato Y., and Scheuer, P.J. (1974). Aplysiatoxin and debromoaplysiatoxin, constituents of the marine mollusk Stylocheilus longicauda. J.Am.Chem.Soc. 96, 2245–2246.
150. Kato Y., and Scheuer. P.J. (1975). The aplysiatoxins. J. Pure.Appl.Chem. 41, 1-14.
151. Kato, Y., and Scheuer, P.J. (1976). The Aplysiatoxins: Reactions with Acid and Oxidants. Pure. Appl. Chem. 48, 29-33.
152. Kaushik, P., and Chauhan, A. (2008). In vitro antibacterial activity of laboratory grown culture of Spirulina platensis. Indian Journal of Microbiology 48, 348-352.
153. Kaushik, P., and Chauhan, A. (2009). Cyanobacteria: Antibacterial Activity. Current science 97, 1378-1379.
154. Kingston, D.G., Rao, M.M., Spittler, T.D., Pettersen, R.C., and Cullen, D.L. (1975). Sesquiterpenes from Flourensia cernua. Phytochemistry 14, 2033-2037.

155
155. Knuebel, G., Larsen, L.K., Moore, R.E., Levine, I.A. and Patterson, G.M.L. (1990). Cytotoxic, antiviral indolocarbazoles from a blue-green alga belonging to the Nostocaceae. Journal of Antibiotics 43, 1236–1239.
156. Koehn, F.E., Lomgley, R.E. and Reed, J.K. (1992). Microcolins A and B, new immunosuppressive peptide from the blue-green algae Lyngbya majuscula. J. Nat. Prod. 55, 613–619.
157. Komárek J. and Anagnostidis, K. (1999). Chroococcales. In Ettl, H., Gärtner, G., Heynig, H., and Mollenhauer, D.,(eds.), Cyanoprokaryota Teil 1. Süsswasserflora von Mitteleuropa 19/1: 1-548. Gustav Fischer Verlag, Jena, Germany.
158. Komárek J. and Anagnostidis, K. (2005). Oscillatoriales. In Büdel, B., Gärtner, G., Krienits, L., Schagerl. M., (eds.), Cyanoprokaryota Teil 2. Süsswasserflora von Mitteleuropa 19/2. Fischer Verl., Jena/Stuttgart/ Lübeck/Ulm.
159. Komárek J., and Anagnostidis, K. (1989). Modern approach to the classification system of cyanophytes. 4. Nostocales. Arch. Hydrobiol. Suppl. 82/Algo. Stud. 56, 247-345.
160. Kreitlow, S., Mundt, S., Lindequist, U. (1999). Cyanobacteria- a potential source of new biologically active substances. J. Biotech. 70, 61-63.
161. Kulik M.M. (1995). The potential for using cyanobacteria (blue- green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur. J. Plant Pathol. 10, 585-599.
162. Laamanen, M.J., Gugger, M.L., Lehtimäki, J.M., Haukka, K., and Sivonen, K. (2001). Diversity of toxic and nontoxic Nodularia isolates (cyanobacteria) and filaments from the Baltic Sea. Appl. Environ. Microbiol. 67, 4638-4647.
163. Lau, A.F., Siedlecki, J., Anleitner, J., Patterson, G.M. L., Caplan, F.R., Moore, R.E. (1993). Inhibition of Reverse Transcriptase Activity by Extracts of Cultured Blue-Green Algae (Cyanophyta). Planta Medica 59, 148–151.
164. Lee, R.E. (1999). Phycology. 3rd edition. Cambridge University Press.
165. Leflaive, J.P., and Ten-Hage, L. (2007). Algal and cyanobacterial secondary metabolites in freshwaters: a comparison of allelopathic compounds and toxins. Freshwater Biology 52, 199-214.
166. Lehtimäki, J., Lyra, C., Suomalainen, S., Sundman, P., Rouhiainen, L., Paulin, L., Salkinoja-Salonen, M., and Sivonen, K. (2000). Characterization of Nodularia strains, cyanobacteria from brackish waters, by genetypic and phenotypic methods. Int. J. Syst. Evol. Microbiol. 50,1043-1053.
167. Lehtimaki, J., Moisander, P., Sivonen, K., and Kononen, K.(1997). Growth, nitrogen fixation and nodularin production by two Baltic Sea cyanobacteria. Appl. Environ. Microbiol. 63, 1647–1654.
168. Liang,J., Richard E. Moore, Eric D. Moher, John E. Munroe, Rima S. Al-awar, David A. Hay, David L. Varie, Tony Y. Zhang, James A. Aikins, Michael J. Martinelli, Chuan Shih, James E. Ray, Lowell L. Gibson, Vasu Vasudevan, Lisa Polin, Kathryn White, Juiwanna Kushner, Chiab Simpson, Susan Pugh and Thomas H. Corbett. (2005). Cryptophycins-309, 249 and other cryptophycin analogs: Preclinical efficacy studies with mouse and human tumors. Investigational New Drugs 23, 213-224.
169. Liebeke, M., Ariane Wunder, A., and Lalk, M. (2010). A rapid microwave-assisted derivatization of bacterial metabolome samples for gas chromatography/mass spectrometry analysis. Analytical Biochemistry 401, 312-314.
170. Lin, Y.,Schiavo, S.,Orjala, J.,Vouros, P., and Kautz, R. (2008). Microscale LC-MS-NMR Platform Applied to the Identification of Active Cyanobacterial Metabolites. Anal. Chem. 80, 8045-8054.

156
171. Lincoln, R.A., Strupinski, K., and Walker, J.M. (1996). The use of Artemia nauplii (brine shimp larvae) to detect toxic compounds from microalgal cultures. Int. J. Pharmacog. 34, 384- 389.
172. Linington, R.G., Edwards, D.J., Shuman, C.J., McPhail, K. L., Matainaho, T., and Gerwick, W.H. (2008). Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 71, 22-27.
173. Linington, R.G., González, J., Ureña, L.D., Romero, L.I., Barrίa, E.O., and Gerwick, W.H. (2007). Venturamides A and B: Antimalarial constituents of the Panamanian marine cyanobacterim Oscillatoria sp. J. Nat. Prod. 70, 397-401.
174. Luesch, H., Richard E. Moore, Valerie J. Paul, Susan L. Mooberry, and Thomas H. Corbett. (2001). Isolation of Dolastatin 10 from the Marine Cyanobacterium Symploca Species VP642 and Total Stereochemistry and Biological Evaluation of Its Analogue Symplostatin 1. J. Nat. Prod. 64, 907–910
175. Luesch, H., Yoshida, W.Y., Moore, R.E., Paul, V.J., Mooberry, S.L. and Corbett, T.H. (2002). Symplostatin 3, a new dolastatin 10 analogue from the marine cyanobacterium Symploca sp. VP452. J. Nat. Prod. 65, 16–20.
176. Lyra, C., Suomalanen, S., Gugger, M., Vezie, C., Sundman, P., Paulin, L., and Sivonen, K. (2001). Molecular characterization of planktic cyanobacteria of Anabaena, Aphanizomenon, Microcystis and Planktothrix genera. Int. J. Syst. Evol. Microbiol. 51, 513-526.
177. MacMillan, J. B., Ernst-Russel, M. A., de Ropp, J. S., Molinski, T. F. (2002). Lobocyclamides A-C, lipopeptides from a Cryptic cyanobacterial mat containing Lyngbya confervoides. J. Org. Chem. 67, 8210-5.
178. Madigan, M.T., Martinko, J.M., and Parker, J., (2003). Brock. Biology of microorganisms. 10th edition. Pearson Education. Upper Saddle River, New Jersey.
179. Magarvey, N. A., Beck, Z. Q., Golakott, T., Ding, Y., Huber, U., Hemscheidt, T. K., Abelson, D., Moore, R.E. and Sherman, D.H. (2006). Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins, potent anti-cancer agents from Nostoc cyanobionts. ACS Chem. Biol. 1, 766– 779.
180. Manen, J.F., and Falquet, J. (2002). The cpcB- cpcA locus as a tool for the genetic characterization of the genus Arthrospira (cyanobacteria): evidence for horizontal transfer. Int, J. Syst. Evol. Microbiol. 52, 861-867.
181. Martins, R.F., Ramos, M., Herfindal,L., Sousa, J.A., Skarven K., and Vasconcelos V.M. (2008). Antimicrobial and cytotoxic Assessment of Marine cyanobacteria- Synechocystis and Synechococcus. Mar. Drugs 6, 1-11.
182. Mason, C. P., Edwards, K. R., Carlson, R. E., Pignatello, J., Gleason, F. K., Wood, J. M. (1982). Isolation of chlorine-containing antibiotic from the freshwater cyanobacterium Scytonema hofmanni. Science 215, 400-2.
183. Matern, U., Obeer, L., Falchetto, R.A., Erhard, M., Konig, W.A., Herdman, M., and Weckesser, J. (2001). Scytoptolin A and B, cyclic depsipeptides from axenic cultures of Scytonema hofmanni PCC 7110. Phytochemistry 58, 1087-1095.
184. Mazel, D., Houmard, J., Castets, A.M., and Tandeau de Marsac, N. (1990). Highly repetitive DNA-sequences in cyanobacterial genomes. J. Bacteriol. 171, 2755-2761.
185. Mazur, H., and Marcin Plinski, M. (2001). Stability of cyanotoxins, microcystin-LR, microcystin-RR and nodularin in seawater and BG-11 medium of different salinity. Oceanologia 43, 329-339.

157
186. McPhail, K.L., Correa, J., Linington, R.G., González, J., Ortega-Barría,E.., Capson, T.L., and Gerwick, W.H.(2007). Antimalarial Linear Lipopeptides from a Panamanian Strain of the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 70, 984–988.
187. Medina, R.A., Goeger, D.E., Hills, P., Mooberry, S.L., Huang, N., Romero, L.I., Ortega-Barria, E., Gerwick, W.H., and McPhail, K.L. (2008). Coibamide A, a potent antiproliferative cyclic depsipeptide from the panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 130, 6324–6325.
188. Meissner, K., Dittmann, E., Borner, T. (2006). Toxic and non-toxic strains of the cyanobacterium Microcystis aeruginosa contain sequences homologous to peptide synthetase genes. FEMS Microbiol Lett 135, 295-303.
189. Mita, A.CA.C., Hammond, L.A., Bonate, P.L., Weiss, G., McCreery, H., Syed, S., Garrison, M.,Chu, Q.S., DeBono, J.S., Jones, C.B., Weitman, S., and E.K. Rowinsky, E.K. (2006). Phase I and pharmacokinetic study of tasidotin hydrochloride (ILX651), a third-generation dolastatin-15 analogues, administered weekly for 3 weeks every 28 days in patients with advanced solid tumors. Clin. Cancer Res. 12, 5207–5215.
190. Mo, S., Krunic, A., Chlipala, G., Orjala, J. (2009). Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod. 72, 894-899.
191. Moon, S.S., Chen, J.L., Moore, R.E., Patterson, G.M.L. (1992). Calophycin, a fungicidal cyclic decapeptide from the terrestrial blue-green algae Calothrix fusca. J.Org. Chem. 57, 1097-1103.
192. Moore R.E., Blackman, A.J., Cheuk, C.E., Mynderse, J.S., Matsumoto, G.K., Clardy, J., Woodard, R.W., and Craig, J.C. (1984). Absolute stereochemistries of the aplysiatoxins and oscillatoxin A. J.Org.Chem. 49, 2484–2489.
193. Moore, R. E., C. Cheuk, X. Q. G. Yang, G. M. L. Patterson, R. Bonjouklian, T. A. Smitka, J. S. Mynderse, R. S. Foster, N. D. Jones, J. K. Swartzendruber, J. B. Deeter. (1987). Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 52, 1036-43.
194. Moore, R.E., Cheuk, C., and Patterson, G.M.L. (1984). Hapalindoles: new alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Am. Chem. Soc. 106, 6456-6457.
195. Moore, R.E., Corbett, T.H., Patterson, G.L., Valeriote, F.A. (1996). The search for new antitumor drugs from blue-green algae. Curr. Pharm. Des. 2, 317–330.
196. Moore, R.E., Patterson, G.M.L., and Carmichael, W.W. (1988). New Pharmaceuticals from cultured blue-green algae. Memoirs of the California Academy of Science 13, 143-150.
197. Moritz, C., and Hillis, D.M. (1996). Molecular systematics: Context and controversies. In: Hillis, D.M., Moritz, C., Mabele, B.K., (Eds.), Molecular Systematics. Sinauer Associates Inc., Sunderland.1-13.
198. Mundt, S., Kreilow, S., Nowotny, A., and Effmert, U. (2001). Biochemical and pharmacological investigations of selected cyanobacteria. Int. J. Hyg. Environ. Health. 203, 327-334.
199. Mundt, S., Kreitlow, S., and Jansen, R. (2003). Fatty acids with antibacterial activity from the cyanobacterium Oscillatoria redekei HUB 051. J.Appl. Phycol. 15, 263-267.
200. Mur, L.R., Skulberg, O.M., and Utkilen, H. (1999). Cyanobacteria in the environment. In Chorus, I., and Bartram, J. (ed.): Toxic cyanobacteria in water: A guine to their public health consequences, mornitoring and management. E & FN Spon, London, UK, 15-40.
201. Mynderse, J.S., and Moore, R.E. (1978). Toxins from blue-green algae: structures of oscillatoxin A and three related bromine-containing toxins. J.Org.Chem.43, 2301–2303.

158
202. Mynderse, J.S., Moore, R.E., Kashiwagi, M., and Norton, T.R. (1977). Antileukemia activity in the Osillatoriaceae: isolation of Debromoaplysiatoxin from Lyngbya. Science 196, 538-540.
203. Nagai, H., Yasumoto, T., and Hokama, Y. (1997). Manauealides, some of the Causative Agents of a Red Alga Gracilaria coronopifolia poisoning in Hawaii. J.Nat.Prod. 60, 925-928.
204. Nagai, H.,Yukiko, K.,Tsuyoshi, F.,Bryan ,S., and Hokama, Y. (1998). Manauealide C and Anhydrodebromoaplysiatoxin, Toxic Constituents of the Hawaiian Red Alga, Gracilaria coronopifolia. Bioscience, biotechnology, and biochemistry. 62, 1011-1013.
205. Namikoshi, M., and Rinehart, K.L. (1996). Bioactive compounds produced by cyanobacteria. J. Ind. Microbiol. Biotechnol. 17, 373–384.
206. Neilan, B.A., Ditmann, E., Rouhiainen, L., Bass, R.A., Schaub, V., Sivonen, K., Borner, T. (1999). Nonribosomal peptide synthesis and toxigenicity of cyabacteria. Journal of Bacteriology 181, 4089-4097.
207. Neilan, B.A., Jacobs, D., and Goodman, A.E. (1995). Genetic diversity and phylogeny of toxic cyanobacteria determined by DNA polymorphisms within the phycocyanin locus. Appl. Environ. Microbiol. 61, 3875-3883.
208. Nelissen, B., Baere, R.D., Wilmotte, A., and Wachter, R.D. (1996). Phylogenetic Relationships of Nonaxenic Filamentous Cyanobacterial Strains Based on 16S rRNA Sequence Analysis. J. Mol. Evol. 42,194-200.
209. Neuhof, T., Schmieder, P., Preussel, K., Dieckmann, R., Pham. H., Bartl, F., and von Döhren, H. Hassallidin A, a glycosylated lipopeptide with antifungal activity from the cyanobacterium Hassallia sp. J. Nat.Prod. 68, 695-700.
210. Newman, D.J., and Cragg, G.M. (2004). Marine natural products and related compounds in clinical and advanced clinical trials. J Nat Prod. 67, 1216-1238
211. Newman, D.J., and Cragg, G.M. (2007). Natural Products as Sources of New Drugs over the Last 25 Years. J.Nat. Prod. 70, 461-477.
212. Noaman, N.H., Fattah, A., Khaleafa, M., and Zaky, S.H. (2004). Factors affecting antimicrobial activity of Synechococcus leopoliensis. Microbiol. Res. 159, 395-402.
213. Norton, R.S., and Wells, R.J. (1982). A series of chiral polybrominated biindoles from the blue-green marine algae Rivularia firma. Application of 13C NMR spin-lattice relaxation data and 13C-1H coupling constants to structure elucidation. J. Am. Chem. Soc. 104, 3628–3635.
214. O’Keefe, B.K., Smee, D.F., Turpin, J.A., Sucedo, C.J., Gustafson, K.R., Mori, T., Blakeslee, D., Buckheit, R., and Boyd, M.R. (2003). Antimicrob. Agents Chemother. 47, 2518–2525.
215. Olaizola, M. (2003). Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol, Eng. 20, 459-466.
216. Orcutt, K.M., Rasmussen, U., Webb, E.A., Waterbury, J. B., Gundersen, K., and Bergman, B. (2002). Characterization of Trichodesmium spp. By genetic techiques. Appl. Environ. Microbiol. 68, 2236-2245.
217. Oren, A. (2000). Salts and brines. In Whitton, B.A., and Potts, M. (ed.). The ecology of cyanobacteria: Their diversity in time and space. Kluwer Academic Publishers Dordrecht, the Netherlands. 281-306
218. Oren, A., and Tindall, B.J. (2005). Nomenclature of the cyanophyta /cyanobacteria/ cyanoprokaryotes under the International Code of Nomenclature of Prokaryotes. Arch. Hydrobiol. Suppl. 159, Algol Stud. 117, 39-52.
219. Østensvik, Ø., Skuberg, O.M., Underdak, B. and Hormazabal, V. (1998). Antibacterial properties of extracts from selected planktonic cyanobacteria- comparative study of bacterial bioassays. J. Appl. Microbiol. 84, 1117- 1124.

159
220. Otsuka, S., Suda, S., Li, R.,Watanabe, M.,Oyaizu, H., Matsumoto, S., and Watanabe, M.M. (1999). Characterization of morphospecies and strains of the genus Microcystis (Cyanobacteria) for a reconsideration of species classification. Phycol. Res. 47,189-197.
221. Ozdemir, G., Karabay, N. U., Dalay, M. C., and Pazarbasi, B. (2004). Antibacterial Activity of Volatile component and Various Extracts of spirulina plasensis. Phytotherary research 18, 754-757.
222. Panda, D., Himes, R. H., Moore, R. E., Wilson, L. and Jordan, M. A. (1997) Mechanism of action of the unusually potent microtubule inhibitor cryptophycin 1 Biochemistry 36, 12948– 12953.
223. Papendorf, O., König, G.M. and Wright, A.D. (1998). Hirridin B and 2,4-dimethoxy-6 heptadecylphenol, secondary metabolites from the cyanobacterium Phormidium ectocarpi with antiplasmodial activity. Phytochem. 49, 2383–2386.
224. Papke, U., Gross, E. M., and Francke, W. (1997). Isolation, Identification and Determination of the Absolute Configuration of Fischerellin B. A new Algicide from the Freshwater Cyanobacterium Fischerella muscicola (Thuret). Tetrehedron Letters 38, 379-382
225. Park, A., Moore, R.E, Patterson, G.M.L. (1992). Fischerindole L, a new isonitrile from the terrestrial blue-green alga Fischerella muscicola. Tetrahedron Lett. 33, 3257–3260.
226. Patel, S., Keohan, M.L., Saif, M.W., Rushing, D., Baez, L., Freit, K., et al., (2006). Phase II study of intravenous TZT-1027 in patients with advanced or metastatic soft-tissue sarcomas with prior exposure to anthracycline-based chemotherapy. Cancer 107, 2881-2887.
227. Patil, L.S., Kulkarni, M.V., and Puranik, P.R. (2009). Assessment of an antibacterial potential of some indigenously isolated culturable cyanobacterial species. Journal of Pharmacy Research 2, 1116-1119.
228. Patterson, G.M.L., and Boils, C.M. (1995). Regulating of scytophycin accumulation in cultures of Scytonema ocellatum II. Nutrient requirement. Appl. Microbiol. Biotechnol. 43, 692–700.
229. Patterson, G.M.L., and Carmeli, S. (1992). Biological effects of tolytoxin (6-hydroxy-7-O-methyl-scytophycin b), a potent bioactive metabolite from cyanobacteria. Arch. Microbiol. 157, 406-410.
230. Patterson, G.M.L., Baker, K.K., Baldwin, C.L., Bolis, C.M., Caplan, F.R., Larsen, L.K., et al., (1993). Antiviral activity of cultured blue-green algae (Cyanophyta). J.Phycol. 29, 125–130.
231. Patterson, G.M.L., Larsen, L.K., and Moore, R.E. (1994). Bioactive natural products from blue-green algae. J. Appl. Phycol. 6, 151–157.
232. Patterson, GML., Baldwin, C.L., Bolis, C.M., Caplan, F.R., Karuso, H., Larsen, L.K., Levine, I.A., Moore, R.E., Nelson, C.S., Tschappat, K.D., Tuang, G.D., Furusawa,E., Furusawa, S., Norton, T.R., Raybourne. R.B. (1991). Antineoplastic activity of cultured blue-green algae (cyanophyta). J. Phycol. 27. 530-536.
233. Paul, V.J. (2008). Global warming and cyanobacterial harmful algal blooms. In Hudnell, H.K. (ed.). Cyanobacterial harmful algal blooms: State of science and research needs. Springer, New York, USA. 239-257.
234. Pawar, S.T., and Puranik, P. R. (2008). Screening of terrestrial and freshwater halotolerant cyanobacteria for antifungal activities. World Journal of Microbiology and Biotechnology 24. 1019-1025.
235. Pedersén, M., and DaSilva, E.J. (1973). Simple brominated phenols in the blue-green alga Calothrix brevissima West. Planta 115, 83-6.

160
236. Perez, E. A., Hillman, D.W., Fishkin, P.A., Krook, J., Tan, W.W., Kuriakose, P.A., Alberts, S.R., and , S.R. (2005). Phase II trial of dolastatin-10 in patients with advanced breast cancer. Investigational New Drugs 23, 257-261.
237. Pergament, I., and Carmeli, S. (1994). Schizotrin A; a novel antimicrobial cyclic peptide from a cyanobacterium. Tetrahedron Lett. 35, 8473-6.
238. Pettersen, R.C., Cullen, D.L., Spittler, T.D., and Kingston, D.G. (1975). The crystal and Molecular structure of Flourensadiol, a natural product sesquiterpene isolated from a West Texas Shrub. Acta Cryst. B41, 1124
239. Pettit, G.R., Kamano, Y., Herald, C.L., Tuiman, A.A., Boettner, F.E., Kizu, H., Schmidt, J.M., Baczynskyj, L., Tomer, K.B., Bontems, R.J. (1987). The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J. Am. Chem. Soc. 109, 6883–6885.
240. Pignatello, J.J., Porwoll, J., Carlson, R.E., Xavier, A., and Gleason, F.K. (1983). Structure of the antibiotic cyanobacterin, a chlorine-containing γ-lactone from the freshwater cyanobacterium Scytonema hofmanni. J. Org. Chem. 48, 4035-4038.
241. Piorreck, M., Baasch, K.H., Pohl, P. (1984). Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry. 23, 207-216.
242. Portmann, C., Blom, J.F., Gademann, K., and Jüttner, F. (2008) Aerucyclamides A and B: Isolation and Synthesis of Toxic Ribosomal Heterocyclic Peptides from the Cyanobacterium Microcystis aeruginosa PCC 7806. J.Nat.Prod. 71, 1193-1196.
243. Prashantkumar, P., Angadi, S.B., Vidyasagar, G.M. (2006). Antimicrobial activity of blue-green and green algae. Indian J Pharm Sci. 68, 647-8.
244. Prinsep, M.R., Thomson, R.A., West, M.L. and Wylie, B.L. (1996). Tolypodiol, an antiinflammatory diterpenoid from the cyanobacterium Tolypothrix nodosa. J.Nat.Prod. 59, 786–788.
245. Queshi, M.A., Kidd, M.T., and Ali, R.S. (1995). Spirulina platensis extract enhances chicken macrophage functions after in vitro exposure. Journal of Nutritional Immunology 3, 35–45.
246. Quinn, R.J., Taylor, C., Suganuma, M., and Fujiki, H. (1993). The conserved acid binding domain model of inhibitors of protein phosphatases 1 and 2A: molecular modelling aspects. Bioorganic and Medicinal Chemistry Letters 3, 1029–1034.
247. Qureshi, M.A., and Ali, R.A. (1996). Spirulina platensis exposure enhances macrophage phagocytic function in cats. Immunopharmacology and Immunotoxicology 18, 457–463.
248. Qureshi, M.A., Garlich, J.D., and Kidd, M.T. (1996). Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacology and
Immunotoxicology 18, 465–476.
249. Ramaswamy, A.V., Flatt, P.M., Edwards, D.J., Simmons, T.L., Han B., and Gerwick, W.H. (2006). The secondary metabolites and biosynthetic gene clusters of marine cyanobacteria. Applications in biotechnology. In: P. Proksch and W.E.G. Muller, Editors, Frontiers in Marine
Biotechnology, Horizon Bioscience, 175–224.
250. Rantala, A., Fewer, D.P., Hisbergue, M., Rouhiainen, L., Vaitomaa, J., Borner, T., Sivonen, K. (2004). Phytogenetic evidence for the early evolution of microcystin synthesis. Proc Natl Acad Sci USA 101, 568-573.
251. Rao, M., Malhotra, S., Fatma, T., Rattan, A. 2007. Antimycobacterial activity from cyanobacterial extracts and phytochemical screening of methanol extract of Hapalosiphon. Pharma. Biol. 45. 88-93.

161
252. Rapala, J., Sivonen, K., Luukkainen, R., and Niemelä, S.I. (1993). Anatoxin-a concentration in Anabaena and Aphanizomenon under different environmental conditions and comparison of growth by toxic and non-toxicAnabaena-strains — a laboratory study. J. Appl. Phycol. 5, 581-591.
253. Rapala, J., Sivonen, K., Lyra, C., and Niemela, S.I. (1997). Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli, App. Environ. Microbiol. 63, 2206–2212.
254. Rasmussen, U., and Svenning, M.M. (1998). Fingerprinting of Cyanobacteria Based on PCR with Primers Derived from Short and Long Tandemly Repeated Repetitive Sequences. Appl. Environ. Microbiol. 64, 265-272.
255. Rastogi, R.P., and Sinha, R.P. (2009).Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnology Advances 27, 521-539.
256. Raveh, A., and Carmeli, S. (2007). Antimicrobial ambiguines from the cyanobacterium Fischerella sp. Collected in Israel. J. Nat. Prod. 70, 196-201.
257. Ray, A., Okouneva, T.,Manna, T.,Miller, H. P.,Schmid, S.,Arthaud, L.,Luduena, R., Jordan., M.A., and Wilson, L. (2007). Mechanism of Action of the Microtubule-Targeted Antimitotic Depsipeptide Tasidotin (Formerly ILX651) and Its Major Metabolite Tasidotin C-Carboxylate. Cancer Res. 67, 3767-3776.
258. Reichelt, J.L., and Borowitzka, M.A. (1984). Antibiotics from algae: results of a large scale screening programme. Hydrobiologia 116/117, 158–168.
259. Reisser, W. (2000). Biotechnological potentials of aeroterrestrial algae. Abtract Book 4th European Workshop. Biotechnology of Microalgae, May 2000 Bergholz-Rehbrücke, Germany.
260. Repka, S., Mehtonen, J., Vaitomaa, J., Saari, L., Sivonen,K. (2001). Effects of nutrients on growth and nodularin production of Nodularia strain GR8b. Microb Ecol. 42, 606-613.
261. Reshef, V., Mizrachi, E., Maretzki, T., Silberstein, C., Loya, S., Hizi, A., Carmeli, S. (1997). New acylated sulfoglycolipids and digalactolipids and related known glycolipids from cyanobacteria with a potential to inhibit the reverse transcriptase of HIV-1. J. Nat. Prod. 60, 1251-1260.
262. Reynolds, C.S. (1987). Cyanobacterial water- blooms. Adv. Bot. Res.13, 67-143.
263. Richman, D.D. (1996). HIV therapeutics. Science 272, 1886–1888.
264. Rickards, R.W., Rothschild, J.M., Willis, A.C., de Chazal, N.M., Kirk, J., Saliba, K.J., Smith, G.D. (1999). Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron, 55, 13513-13520.
265. Rinehart, K.L., Jr., P. D. Shaw, L. S. Shield, J. B. Gloer, G. C. Harbour, M. E. S. Koker, D. Samain, R. E. Schwartz, A. A. Tymiak, D. L. Weller, G. T. Carter, M. H. G. Munro, R. G. Hughes, Jr., H. E. Renis, E. B. Swynenberg, D. A. Stringfellow, J. J. Vavra, J. H. Coats, G. E. Zurenko, S. L. Kuentzel, L. H. Li, G. J. Bakus, R. C. Brusca, L. L. Craft, D. N. Young and J. L. Connor. (1981). Marine natural products as sources of antiviral, antimicrobial, and antineoplastic agents. Pure and Applied Chemistry 53, 795–817.
266. Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M., and Stanier, R.Y. (1979). Generic assignments. Strain histories and properties of pure cultures of cynobacteria. J. Gen. Microbiol. 111, 1-61.
267. Rohr, J. (2006). Cryptophycin Anticancer Drugs Revisited. ACS Chem. Biol. 1, 747–750.

162
268. Rohrlack, T., Edvardsen, B., Skulberg, R., Halstvedt, C.B., Utkilen, H.C., Ptacnik, R., and Skulberg, O.M. (2008). Oligopeptide chemotypes of the toxic freshwater cyanobacterium Planktothrix can form subpopulations with dissimilar ecological traits. Limnol Oceanogr. In press
269. Sailer, M., Helms, G.L., Henkel, T., Niemczura, W.P., Stiles, M.E., and Vederas, J.C. (1993). 15 N- and 13 C-labelled media from Anabaena sp. for universal isotopic labeling of bacteriocins: NMR resonance assignments of leucocin A from Levconotoc gelidum and nisin A from Lactococcus lactis. Biochemistry 32, 310–318
270. Sakamoto,T .,Higashi, S., Wada, H., Murata, N., Bryant, D.A. (1997). Low-temperature-induced desaturation of fatty acids and expression of desaturase genes in the cyanobacterium Synechococcus sp. PCC 7002. FEMS Microbiology Letters 152, 313-320.
271. Saleem, M., Nazir, M., Ali, M.S., Hussain, H., Lee, Y.S., Riaz, N., and Jabbar, A. (2010). Antimicrobial natural products: an update on future antibiotic drug candidates. Nat. Prod. Rep. 27, 238-254.
272. Satish, N., Krugman, T., Vinogradova, O.N., Nevo, E., and Kashi, Y. (2001). Genome evolution of the cyanobacterium Nostoc linckia under sharp microclimatic divergence at "evolution Canyon". Microb Ecol. 42, 306-316.
273. Schawartz, R.E., Hirsch, C.F., Sigmund, J.M., and Pettibone, D.J. (1989). Haploindolone compounds as vassopressin antagonists, USA Patent Number 4803217.
274. Schlegel, I., Doan, N.T., de Chazal, N., Smith, G.D. (1999). Antibiotic activity of new cyanobacterial isolates from Australia and Asia against green algae and cyanobacteria. J. Appl. Phycol 10, 471–479.
275. Schopf, J. W. (2000). The Fossil Record: Tracing the Roots of the Cyanobacterial Lineage. In: Whitton, B. A., Potts, M., (Eds.), The Ecology of Cyanobacteria. Their Diversity in Time and Space. Kluwer Academic Publishers Dordrecht. 13-35
276. Schwartz, R.E., Hirsch, C.F., Sesin, D.F., Flor, J.E., Chartrain, M., Fromtling, R.E., Harris, G.H., Salvatore, M.J., Liesch, J.M., Yudin, K. (1990). Pharmaceuticals from cultured algae. Journal of Industrial Microbiology 5, 113-124.
277. Schwartz, R.E., Hirsch, C.F., Sigmund, J.M., Pettibone, D.J. (1989). Hapalindolinone compounds as vassopressin antagonists, US Patent No. 4,803, pp. 217–223.
278. Sessa, C., Weigang-Kohler, K., Pagani, O., Greim, G., Mora, O., De Pas, T., Burgess, M., Weimer, I. and Johnson, R. (2002). Phase I and pharmacological studies of the cryptophycin analogue LY355703 administered on a single intermittent or weekly schedule. Eur. J. Cancer 38, 2388– 2396.
279. Shanab, S.M.M. (2007). Bioactive Allelo- chemical Compounds from Oscillatoria Species (Egyptian Isolates). Int. J. Agric. Biol. 9, 617-621.
280. Shimizu, Y. (2003). Microalgal metabolites. Curr. Opin. Microbiol. 6, 236–243.
281. Sielaff, H., Christiansen, G., and Schwecke, T. (2006). Natural products from cyanobacteria: Exploiting a new source of drug discovery. IDrugs 9, 119- 127.
282. Silva, P. G., Silva, H. J. (2007). Effect of mineral nutrients on cell growth and self-flocculation of Tolypothrix tenuis for the production of a biofertilizer. Bioresource Technology 98, 607-611.
283. Simmons, T.L., Andrianasolo, E., McPhail, M., Flatt, P., Gerwick, W.H. (2005). Marine natural products as anticancer drugs. Mol. Cancer Ther. 4, 333-342.
284. Simmons, T.L., Engene, N., Ureña, L.D., Romero, L.I., Barrίa, E.O., Gerwick, L., and Gerwick, W.H. (2008). Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium Oscillatoria nigro-viridis. J. Nat. Prod. 71, 1544-1550.

163
285. Simmons, T.L., McPhail, K.L., Ortega-Barria, E., Mooberry, S.L., Gerwick, W.H. (2006). Belamide A, a new antimitotic tetrapeptide from a Panamanian marine cyanobacterium. Tetrahedron Lett. 47, 3387–3390
286. Simonin, P., Jürgens,U.J., and Rohmer, M. (1992). 35-O-β-Amino-deoxyglucopyranosyl bacteriohopanetetrol, a novel triterpenoid of the hopane series from the cyanobacterium synechocystis sp. PCC 6714. Tetrahedron Lett. 33, 3629-3632.
287. Singh, S., Kate, B.N., and Benerjee, U.C. (2005). Bioactive compounds from cyanobacteria and microalgae: an overview. Crit. Rev. Biotechnol. 25, 73–95.
288. Sivonen, K. (1990). Effects of light, temperature, nitrate, orthophosphate and bacteria on growth of and hepatotxin production by Oscillatoria agardhii strains. Appl. Environ. Microbiol. 56, 2658–2666.
289. Sivonen, K., and Boerner, T. (2008). Bioactive compounds produced by cyanobacteria. Edited by Antonia, H., and Enrique, F. 159-197.
290. Sivonen, K., Jouni, J., Matti, W., Perttu, P., Ove, D.S., Lars, H. (2007). Bioactive cyclic peptide. PCT Int. Appl.
291. Skulberg, O.M. (2000). Microalgae as a source of bioactive molecules-experience from cyanophyte research. J. Appl. Phycol. 12,341–348.
292. Smith, C. D., Zhang, X., Mooberry, S. L., Patterson, G. M. and Moore, R. E. (1994). Cryptophycin: a new antimicrotubule agent active against drug-resistant cells. Cancer Res. 54, 3779– 3784.
293. Smith, G. D., and Doan, N. T. (1999). Cyanobacterial metabolites with bioactivity against photosynthesis in cyanobacteria, algae and higher plants. J. Appl. Phycol. 11, 337-44.
294. Smith, J. Boyer, L., G. L., and Zimba, P. V. (2008). Impacts of noxious and odorous cyanobacterial metabolites on aquaculture systems. Aquaculture 280, 5-20.
295. Smitka, T.A., Bonjouklian, R., Doolin, L., Jones, N.D., Deeter, J.B., Yoshida, W.Y., Prinsep, M.R., Moore, R.E., Patterson, G.M.L. (1992). Ambiguine isonitriles, fungicidal hapalindole-type alkaloids from three genera of blue-green algae belonging to the Stigonemataceae. J. Org. Chem. 57, 857–861.
296. Smitka, T.A., Bonjouklian, R., Doolin, L., Jones, N.D., Deeter, J.B., Yoshida, W.Y., Prinsep, M.R., Moore, R.E., and Patterson, G.M.L. (1992). Ambiguine isonitriles, fungicidal hapalindole-type alkaloids from three genera of blue-green algae belonging to the Stigonemataceae. J. Org. Chem. 57, 857–861.
297. Soltani, N., Khavari- Nejad, R.A., Yazdi, M.T., Shokravi, S., and Fernández-Valiente E. (2005). Screening of soil cyanobacteria for antifungal and antibacterial activity. Pharm Biol. 43, 455-459.
298. Soltani, N., Zarrini, G., Ghasemi. Y., Shokravi, Sh., and Baftehchi, L. (2007). Characterization of a soil Cyanobacterium Fischerella sp. FS 18 under NaCl Stress. Journal of Biological Sciences 7, 931-936.
299. Spolaore, P., Joannis-Cassan, C., Duran, E., and Isambert, A. (2006). Commercial applications of microalgae. J. Biosci. Bioeng. 101, 87-96.
300. Srivastava, V. C., Manderson, G. J., and Bhamidimarri, R. (1999). Inhibitory metabolites production by the cyanobacterium Fischerella muscicola. Microbiol Res. 153, 309–317
301. Stam, W.T. (1980). Relationships between a number of filamentous blue- green algal strains (Cyanophyceae) revealed by DNA-DNA hybridization. Algol. Stud. 25, 351-374.
302. Stam, W.T., and Stulp, B.K. (1988). New taxonomic methoads:DNA/DNA hybridization. Methods Enzymol. 167, 125-132.

164
303. Stanier, R. Y., and Cohen-Bazire, G. (1977). Phototrophic Prokaryotes: The Cyanobacteria. In M. P. Starr, J. L. Ingraham, & A. Balows (Eds.), Annual Review of Microbiology. Palo Alto, CA: Annual Reviews Inc. 225-274.
304. Stewart, I., Carmichael, W.W., Sadler, R., McGregor, G.B., Reardon, K., Eaglesham, G.K., Wickramasinghe, W.A., Seawright, A.A., and Shaw, G.R. (2009). Occupational and environmental hazard assessments for the isolation, purification and toxicity testing of cyanobacterial toxins. Environmental. Health 8, 52.
305. Stratmann, K., Moore, R.E., Bonjouklian, R., Deeter, J.B., Patterson, G.M.L. Shaffer, S., Smith, C.D., Smitka, T.A. (1994). Welwitindolinones, Unusual Alkaloids from the Blue-Green Algae Hapalosiphon welwitschii and Westiella intricata. Relationship to Fischerindoles and Hapalinodoles. J.Am.Chem.Soc. 116, 9935-9942.
306. Suganuma, M., Fujiki, H., Tahira, T., Cheuk, C., Moore, R.E., and Sugimura, T. (1984). Estimation of tumor promoting activity and structure-function relationships of aplysiatoxins. Carcinogenesis 5, 315-318.
307. Svircev, Z., Cetojevic-Simin, D., Simeunovic, J., Karaman, M., and Stojanovic, D. (2008). Antibacterial, antifungal and cytotoxic activity of terrestrial cyanobacterial strains from Serbia. Sci. China Ser. C- Life. Sci. 51, 941-947.
308. Tamura, K., Nakagawa, K., Kurata, T., Satoh, T., Nogami, T., Takeda, K., Mitsuoka, S., Yoshimura, N., Kudoh, S., Negoro, S., and Fukuoka, M. (2007). Phase I study of TZT-1027, a novel synthetic dolastatin 10 derivative and inhibitor of tubulin polymerization, which was administered to patients with advanced solid tumors on days 1 and 8 in 3-week courses. Cancer Chemother Pharmacol (in press).
309. Tan, L. T. (2007). Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 68, 954-979.
310. Tan, L.K., Chang, Y. Y., and Ashootosh, T. (2008). Besarhanamides A and B from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 69, 2067-2069.
311. Tan, L.T. (2006). Biomedical potential of marine cyanobacteria. Journal of Coastal Development 9, 129-136.
312. Taori, K., Paul, V. J., and Luesch, H. (2008). Kempopeptins A and B, serine protease inhibitors with different selectivity profiles from a marine cyanobacterium Lyngbya sp. J. Nat. Prod. 71, 1625-1629.
313. Taori, K., Paul, V. J., and Luesch, H. J. (2008). Kempopeptins A and B, Serine Protease Inhibitors with Different Selectivity Profiles from a Marine Cyanobacterium, Lyngbya sp. J. Nat. Prod. 71, 1625-1629.
314. Taton, A., Grubisic, S., Ertz, D., Hodgson, D.A., Piccardi, R., Biondi, N., Tredici, M.R., Mainini, M., Losi, D., Marinelli, F., and Wilmotte, A. (2006). Polyphasic study of antarctic cyanobacterial strains. J Phycol. 42, 1257–1270.
315. Teneva, I., Dzhambazov, B., Mladenov, R., and Schirmer, K. (2005). Molecular and phylogenetic characterization of Phormidium species (Cyanoprokaryota) using the cpc B-IGS-cpcA locus. J.Phycol. 41,188-194.
316. Thajuddin, N., and G. Subramanian, G. (2005). Cyanobacterial biodiversity and potential applications in biotechnology. Curr. Sci. 89, 47–57.
317. Tidgewell, K., Clark, B.R., Gerwick, W.H. (2009).The natural products chemistry of cyanobacteria. In: Mander LN, Liu HW (eds). Comprehensive Natural Products Chemistry II, Volume 8. London: Pergamon Press.

165
318. Todorova, AK., Jüttner, F., Linden, A., Plüss, T., and von Philipsborn W. (1995). Nostocyclamide: a new macrocyclic, thiazole-containing allelochemical from Nostoc sp. 31(Cyanobacteria). J. Org. Chem. 60, 7891-7895.
319. Tomitani, A., Knoll, A.H., Cavanaugh, C.M., and Ohno, T. (2006). The evolutionary diversification of cyanobacteria: Molecular-phylogentic and paleontological perspectives. PNAS. 103, 5442-5447.
320. Tripathi, A., Puddick, J., Prinsep, M.R., Lee, P.P.F., and Tan, L.T. (2009). Hantupeptin A, a Cytotoxic Cyclic Depsipeptide from a Singapore Collection of Lyngbya majuscula. J. Nat. Prod. 72, 29–32.
321. Tripathi, A., Puddick, J., Prinsep, M.R., Lee, P.P.F., Tan, L.T. (2009). Hantupeptin A, a cytotoxic cyclic depdipeptide from a Singapore collection of Lyngbya majuscula. J. Nat. Prod. 72, 29-32.
322. Turk, B. (2006). Targeting proteases: successes, failures and future prospects. Nat Rev. Drug. Discov. 5, 785-799
323. Utkilen, H., and Giolme, N. (1995). Ironstimulated toxin production in Microcystis
aeruginosa. Appl. Environ. Microbiol. 36, 797–800.
324. Van den Hoek, C., Mann, D.G. and Jahns, H.M. (1995). Algae: an introduction to phycology. Cambridge: Cambridge University Press.
325. Van Wagoner, R.M.,Drummond, A.K., and Wright, J.L.C (2007).Biogenetic diversity of cyanobacterial metabolites. Adv. Appl. Microbiol. 61, 89–217
326. Vepritskii, A.A., Gromov, B.V., Titota, N.N., and Mamkaeva, K.A. (1991). Production of the antibiotic-algicide cyanobacterin LU-2 by a filamentous cyanobacterium Nostoc sp. Mikrobiologia 60, 21–25,
327. Villa, F.A. Lieske, K. and Gerwick, L. (2010). Selective MyD88-dependent pathway inhibition by the cyanobacterial natural product malyngamide F acetate. Eur.J.Pharmacol. 629, 140.
328. Vo, H., and Ho, S.H. (2004). Cyanobacteria in the rice fields of Dak Lak province. Proceedings, the 2005
th National conference on life sciences Thai Nguyen University, Science and
Technics publishing House September 23, 2004, 88-91.
329. Vo, H., Ho, S.H., Le, N.T., and Duong, D.T. (2006). The result of isolating some species of Heterocyst cyanobacteria from agricultural soil of Dak Lak province. Collection of Scientific reports of Vietnamese National university 1, 57-63,
330. Volk, R.B. (2006). Antialgal activity of several cyanobacterial exometabolites. J. Appl. Phycol. 18, 145–151.
331. Volk, R.B. and Furkert, F. H. (2006). Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiological Research 161, 180-186.
332. Walsby, A. E. (1994). Gas vesicles. Microbiol. Rev. 58, 94- 144.
333. Wase, N.V., and Wright, P.C. (2008). Systems biology of cyanobacterial secondary metabolite production and its role in drug discovery. Exp. Opin. Drug. Discov. 3, 903–929.
334. Watanabe, J., Minami, M., Kobayashi, M., Natsume, T., Watanabe, J., Horiuchi, T., and Kobayashi, M. (2006). Antitumor activity of TZT-1027 (Soblidotin). Anticancer Res. 26, 1973–1981.
335. Watanabe, M.M (2005). Freshwater culture media. In: Algal culturing techniques. Andersen, R. A., Elservier Academic Press: 13-20.

166
336. Welker, M., and VonDöhren, H. (2006). Cyanobacterial peptides – Nature's own combinatorial biosynthesis. FEMS Microbiol.Rev. 30,530-563.
337. Welker, M., and von Döhren, H. (2006). Cyanobacterial peptides-Nature′s own combinatorial biosynthesis. FEMS Microbiol. Rev. 30, 530-563.
338. Whitton, B.A. and Potts, M. (2000). Introduction to the cyanobacteria. In: Whitton, B.A., Potts, M., (Eds.), The Ecology of Cyanobacteria. Their Diversity in Time and Space. Kluwer Academic Publishers Dordrecht. 1-11.
339. Wiegand, C., and Pflugmacher, S. (2005). Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 203, 201–218.
340. Wift, P., Reeves, J.T., and Day, B.W. (2004). Chemistry and Biology of Curacin A. Curr. Pharm. Design.10, 1417-37.
341. Wilmotte, A. (1994). Molecular evolution and taxonomy of cyanobacteria. In: Bryant, D.A., (Ed.), The Molecular Biology of Cyanobacteria, Kluwer, Dordrecht. 1-25.
342. Wilmotte, A., and Golubic, S. (1991). Morphological and genetic criteria in the taxonomy of Cyanobacteria/ Cyanobacteria. Algo. Stud. 64, 1-24.
343. Wilmotte, A., and Herdman, M. (2001). Phylogenetic relationships among the cyanobacteria based on 16S RRNA sequences. In: Boone, D.R. and Castenholz R.W. (Eds.) Bergey's Manual of Systematic Bacteriology. Second edition, Springer, New York. 487-493.
344. Wilmotte, A., Stam, W. and Demoulin, V. (1997). Taxonomic study of marine oscillatoriacean strains (Cyanophyceae, Cyanobacteria) with narrow trichomes. III. DNA-DNA hybridization studies and taxonomic conclusions. Algol Stud. 87, 11-28.
345. Woese, C. R. (1987). Bacterial evolution. Microbiol Rev. 51, 221–271.
346. Woese, C. R., Sogin, M., Stahl, D., Lewis, B.J., and Bonen, L. (1976). A comparison of the 16S ribosomal RNAs from mesophilic and thermophilic bacilli: Some modifications in the Sanger method for RNA sequencing. J. Mol. Evol. 7, 197- 213.
347. Xiong, C., D’Keefe, B. R., Botos, I., Wlodawer, A., Memahon, S. B. (2006) Overexpression and purification of Scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium . Protein expression and purification 46, 233-239
348. Xiong, S., Fan, J., and Kitazato, K., (2010). The antiviral protein cyanovirin-N: the current state of its production and applications. Appl Microbiol Biotechnol. 86, 805-812.
349. Zainuddin, E.N., Jansen, R., Nimtz, M., Wray, V., Preisitsch, M., Lalk, M., Mundt, S. (2009). Lyngbyazothrins A-D, antimicrobial cyclic undecapeptides from the cultured cyanobacterium Lyngbya sp. J. Nat. Prod. 72, 1373-8.
350. Zainuddin, E.N., Mentel, R., Wray, V., Jansen, R., Nimtz, M., Lalk, M., Mundt, S. (2007). Cyclic depsipeptides, ichthyopeptins A and B, from Microcystis ichthyoblabe. J. Nat. Prod. 70, 1084-8.
351. Zang, L. H., Longley, R., and Koehn, F.E. (1997). Antiproliferative and immunosuppressive properties of microcolin A, a marine-derived lipopeptide. Life Sci. 60, 751-762
352. Whitton, B.A. and Potts, M. (2000). Introduction to the cyanobacteria. In: Whitton, B.A., Potts, M., (Eds.), The Ecology of Cyanobacteria. Their Diversity in Time and Space. Kluwer Academic Publishers Dordrecht. 1-11.
353. Wiegand, C., and Pflugmacher, S. (2005). Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 203, 201–218.
354. Wift, P., Reeves, J.T., and Day, B.W. (2004). Chemistry and Biology of Curacin A. Curr. Pharm. Design.10, 1417-37.

167
355. Wilmotte, A. (1994). Molecular evolution and taxonomy of cyanobacteria. In: Bryant, D.A., (Ed.), The Molecular Biology of Cyanobacteria, Kluwer, Dordrecht. 1-25.
356. Wilmotte, A., and Golubic, S. (1991). Morphological and genetic criteria in the taxonomy of Cyanobacteria/ Cyanobacteria. Algo. Stud. 64, 1-24.
357. Wilmotte, A., and Herdman, M. (2001). Phylogenetic relationships among the cyanobacteria based on 16S RRNA sequences. In: Boone, D.R. and Castenholz R.W. (Eds.) Bergey's Manual of Systematic Bacteriology. Second edition, Springer, New York. 487-493.
358. Wilmotte, A., Stam, W. and Demoulin, V. (1997). Taxonomic study of marine oscillatoriacean strains (Cyanophyceae, Cyanobacteria) with narrow trichomes. III. DNA-DNA hybridization studies and taxonomic conclusions. Algol Stud. 87, 11-28.
359. Woese, C. R. (1987). Bacterial evolution. Microbiol Rev. 51, 221–271.
360. Woese, C. R., Sogin, M., Stahl, D., Lewis, B.J., and Bonen, L. (1976). A comparison of the 16S ribosomal RNAs from mesophilic and thermophilic bacilli: Some modifications in the Sanger method for RNA sequencing. J. Mol. Evol. 7, 197- 213.
361. Xiong, S., Fan, J., and Kitazato, K., (2010). The antiviral protein cyanovirin-N: the current state of its production and applications. Appl Microbiol Biotechnol. 86, 805-812.
362. Zang, L. H., Longley, R., and Koehn, F.E. (1997). Antiproliferative and immunosuppressive properties of microcolin A, a marine-derived lipopeptide. Life Sci. 60, 751-762

168
Acknowledgments
This PhD thesis work was carried out at Institute of Pharmacy, Department of
Pharmaceutical Biology, Ernst-Moritz-Arndt University Greifswald, Germany with
help from many individuals and organizations.
First and foremost, I would like to express my deepest gratitude and thanks to
my supervisor, PD. Dr. Sabine Mundt who gave me the great opportunity to be
involved in cyanobacterial natural product research in her group with for generous
support, continuous encouragement, direct expert guidance and admirable advice
throughout my work. She will always have a special place in my memories.
I wish to express my special gratitude to Prof. Dr. Ulrike Lindequist for
giving me an opportunity to study in her Institute, for guiding, supporting and
providing working facilities during my work, and for partial financial supports in
finishing my study.
I am deeply indebted to Dr. Victor Wray and Dr. Rolf Jansen, Helmholt
Center for Infection Research Braunschweig for structure elucidation of the isolated
natural products. Also I would like to express my sincere thanks to Dr. Victor Wray
for giving me a chance to be in his laboratory and for his guidance, fruitful discussion,
constructive advises, and valuable moral support in finishing this thesis.
I would like to express my great thanks to Dr. Dang Diem Hong in Institute of
Biotechnology, Vietnam Academy of Science and Technology, Ha Noi, Vietnam; Dr.
Ho Sy Hanh in Pedagogical College, Dak Lak, Vietnam; M.Sc. Pham Huu Tri in
Institute of Oceanography, Vietnam Academy of Science and Technology, Nha
Trang, Vietnam for providing cyanobacterial strains.
I would like to express my sincere thanks to Dr. Kristian Wende in our
institute for cytotoxic tests and Dr. Martina Wurster in our institute for identification
of the volatile components and fatty acids of some my samples together with their
kind help and useful suggestions during my work.
I would like to express my great thanks to Mrs. H. Bathrow not only for her
help, advise, encouragement during culture of cyanobacteria, but also for moral
support, nice atmosphere, and familiar situation during my stay in Greifswald.
My special thanks come to PD. Dr. Michael Lalk in our institute for his help in
recording HRMS and NMR spectra of some compounds and for supporting me as

169
well as spending time on my questions. The kind help of Mr. Dipl. Phys. K.H.
Lichtnow in the aspect of computer is also appreciated.
I would like to express my gratitude to Prof. Dr. Le Tran Binh, Dr. Jörn
Kasbohm, Dr. Le Thi Lai (Join Graduate Education Program) and Dr. Nguyen Van
Ngu (Ministry of Education and Training of Vietnam) for their kindness, generous
considerations, precious advices, unwavering support, and continuous encouragement
and for giving me a chance to study in Greifswald.
My special thanks to Prof. Dr. Ramzi A.A. Mothana in Faculty of Pharmacy,
Sana’a-University, Yemen; Prof. Dr. Sahar Hussein in National Research Center,
Egypt; Dr. Wajid Rehman in Department of Chemistry, Hazara University, Pakistan;
Dr. Gerold Lukowski in Institute of Marine Biotechnology, Greifswald, Germany for
their useful discussions and scientific advices to broaden my knowledge.
I wish to thanks all members of Pharmaceutical Biology Department, Institute
of Pharmacy, Greifswald, former and present, for the friendly and comfortable
working atmosphere. Especially, I would like to thanks Mrs. R.Ball, Mrs. Matthias,
Mrs. Fenske, PD. Dr. B. Haertel, M. Preisitsch, E. Puhlmann, W. Poggendorf, B.T.
Huong, M.Harm, S. Blackert, K. Tarman, M. Shushni, A. Hassan, K. Eiden, Z.
Alresly, C. Bäcker for technical assistance, exchanging experiments and nice
discussion related to work and life in Greifswald. Their friendship really helped me to
warm up my time of stay in Greifswald and help me to understand more about the
people and the culture of Germany.
I wish to express my thanks to all members of board of direction of Hong Duc
University and all my colleagues of Natural Sciences faculty and in Department of
Plant, Hong Duc university, my friends and my students in Vietnam and all over the
world for their supporting, sharing and caring.
My deep thanks to all members at the Department of Algal Biotechnology,
Institute of Biotechnology, VAST for their kind help and sharing a nice atmosphere,
and fruitful discussions during my time in this department, especially I would like my
express deepest gratitude to Dr. Dang Diem Hong the leader of this department not
only for providing soil cyanobacterial strains but also for providing me an opportunity
to work in her laboratory and her guidance, valuable support, fruitful discussions, and
constructive advices, in algae culturing techniques and molecular biology of
cyanobacteria.

170
Prof. Dr. Vo Hanh in Vinh University and Dr. Duong Thi Thuy in Institute of
Environmental Technology, VAST, Ha Noi are thanked for their kind help and
fruitful discussion on identification and culturing of cyanobacteria. Also Dr. Nguyen
Huu Dai, the leader of Department of Marine Botany, Institute of Oceanography,
VAST, Nha Trang, Vietnam is thanked for his kind help, support, and fruitful
discussion during my stay in Nha Trang for collecting the marine cyanobacterium
Lyngbya majuscula sample.
Many thanks go to all my friends in Greifswald for their sharing in working
and life during my stay here, especially I would like to thanks Nguyen Hien, K.R.
Baswani for their valuable discussion in chemistry and my deep thanks also to
Nghiem Quynh Huong for her precious help in dissertation formatting.
Special thanks go to the laboratory of the Baltic-Analytics GmbH for
performing GC-MS experiments of some my samples.
I would like to acknowledge the Ministry of Education and Training (MOET),
Vietnam and German Academic Exchange Service (DAAD) for providing me with
the doctoral fellowship. Also my special thanks go to Institute of Marine
Biotechnology (IMAb), Greifswald and Dr. G.Roth from Foreign Students Office,
AAA, for providing me a partial financial support in finishing my study.
Last, but not least, I would like to express my deepest gratitude and thanks to
my mother, my great family, and my husband for their eternal support, continuous
encouragement during my work. Especially to my lovely son, Hoang An, who bring
me happiness, a lot of joys, hopes, and promotion to fulfill this work.

171
Curriculum Vitae
Personal Data
Name: Le Thi Anh Tuyet
Gender: Female
Date of birth: 19 May 1973
Place of birth: Thanh Hoa, Vietnam
Nationality: Vietnamese
Marital status: Married
Educational Background
January 2008 to present Ph.D student at the Institute of Pharmacy, Ernst-Moritz-
Arndt University of Greifswald, Germany
2007 Research internship in Algal Culturing Techniques at
Algal Biotechnology Department, Institute of
Biotechnology, Ha Noi,Vietnamese Academy of Science
and Technology
Oct. 2006-Dec. 2006 Training course on Methods for Natural Products
Isolation at Institute of Pharmaceutical Biology and
Biotechnology, Heinrich-Heine-University, Düsseldorf,
Germany
Oct. 2005-Sept. 2006 Post-graduate training course in Molecular Biology at
Environmental Health Research Institute (IUF), Heinrich-
Heine-University Düsseldorf gGmbH, Düsseldorf,
Germany
Aug. 2005-Sept. 2005 German language course in Carl Duisburg Centren
Dortmund, Germany supported by DAAD
1995-1997 Master of Science (M.Sc.) degree in Biology at Hanoi
University for Teacher’s Training, Vietnam National
University-Hanoi, Vietnam (now is Hanoi National
University of Education). The thesis experiments were
carried at Animal Gene Technology Department, Institute

172
of Biotechnology (IBT), Ha Noi, Vietnamese Academy of
Science and Technology (VAST)
1990-1994 Bachelor of Science (B.Sc.) degree in Biology at Vinh
University, Vietnam
Employment Record
Oct. 1998-Aug. 2005 Worked as a lecturer at Natural Sciences Faculty, Hong
Duc University, Thanh Hoa, Vietnam
Jun. 1997-Sept. 1998 Worked as a teacher at High School in Thanh Hoa,
Vietnam
Sept. 1994-April 1995 Worked as a teacher at High School in Thanh Hoa,
Vietnam

173
List of publications and other scientific achievements 1. Le Thi Anh Tuyet, Victor Wray, Rolf Jansen, Manfred Nimtz, Ho Sy Hanh, Dang
Diem Hong, Sabine Mundt. Daklakapeptin, a new cyclic peptide with antibacterial
activity from the soil cyanobacteria Calothrix javanica and Scytonema ocellatum from
Vietnam (close to submission)
2. Le Thi Anh Tuyet, Victor Wray, Manfred Nimtz, Ho Sy Hanh, Dang Diem Hong,
Sabine Mundt. An extracellular sesquiterpenoid with antibacterial activity from the
soil cyanobacterium Anabaena sp. from Vietnam (close to submission)
3. Le Thi Anh Tuyet, Rolf Jansen, Victor Wray, Martina Wurster, Ho Sy Hanh,
Dang Diem Hong, Sabine Mundt. Antibiotic constituents from the Vietnamese soil
cyanobacterium Westiellopsis sp.VN (close to submission)
4. Wajid Rehman, Amin Badshah, Salimullah Khan and Le Thi Anh Tuyet (2009)
Synthesis, characterization, antimicrobial and antitumor screening of some
diorganotin(IV) complexes of 2-[(9H-Purin-6-ylimino)]-phenol. European Journal of
Medicinal Chemistry 44(10), 3981-3985.
5. Nguyen Van Cuong, Le Thi Anh Tuyet (1997) Initial results of the study on
generating gene transferred fish. Vietnam Journal of Genetics and Applications 2:10-
16.
Poster
1. Le Thi Anh Tuyet and Sabine Mundt. Screening of soil cyanobactria from
Vietnam for antibacterial activity. In "13th International symposium on phototrophic
prokaryotes", August 9-14, 2009, Montreal, Quebec, Canada.

174
Appendix
Appendix 1a: 1H NMR of fraction WF1-3 of Westiellopsis sp. VN

175
Appendix 1b: 1H NMR of fraction WF1-3 of Westiellopsis sp. VN

176
Appendix 2a: 1H NMR of fraction WF1-5 of Westiellopsis sp. VN

177
Appendix 2b: 1H NMR of fraction WF1-5 of Westiellopsis sp. VN

178
Appendix 3a: 1H NMR of fraction WF1-6 of Westiellopsis sp. VN

179
Appendix 3b: 1H NMR of fraction WF1-6 of Westiellopsis sp. VN

180
Appendix 4a: 1H NMR of fraction WF1-8 of Westiellopsis sp. VN

181
Appendix 4b: 1H NMR of fraction WF1-8 of Westiellopsis sp. VN

182
10.00 15.00 20.00 25.00 30.00 35.00 40.00 45.00 50.00 55.00 60.000
200000
400000
600000
800000
1000000
1200000
1400000
1600000
1800000
2000000
2200000
2400000
2600000
2800000
3000000
3200000
3400000
3600000
3800000
4000000
4200000
4400000
4600000
Time-->
Abundance
TIC: 010714.D
Appendix 5: GC-MS spectrum for the fatty acids of fraction MeOH of Westiellopsis sp. VN in n-hexane hydrolysis /derivatization

183
10.00 15.00 20.00 25.00 30.00 35.00 40.00 45.00 50.00 55.00 60.000
200000
400000
600000
800000
1000000
1200000
1400000
1600000
1800000
2000000
2200000
2400000
2600000
2800000
3000000
3200000
3400000
3600000
3800000
4000000
4200000
4400000
4600000
4800000
5000000
5200000
5400000
5600000
5800000
Time-->
Abundance
TIC: 010715.D
Appendix 6: GC-MS spectrum for the fatty acids of fraction MeOH of Westiellopsis sp. VN in MeOH hydrolysis /derivatization

184
Diameter of inhibition zone in mm1
Strain
Cultivation of
cyanobacteri
a
Extract B.s.a S.a.
b E.e.
c
P.a.d
C.m.e
n-hexane ext. 0.0 0.0 0.0 0.0 n.t.
MeOH ext. 14,0 9.0 0.0 0.0 11.0
Batch
EtOAc ext. 18.0 16.0 21.0 0.0 n.t.
n-hexane ext. 0.0 0.0 0.0 n.t. 0.0
MeOH ext. 0.0 0.0 0.0 n.t. 0.0
Anabaena
sp.
Large scale
EtOAc ext. 15.0 16.0 24.0 n.t. 16.0
n-hexane ext. 0.0 0.0 0.0 0.0 n.t.
MeOH ext. 11.0 13.0 8.0 0.0 n.t.
Batch
EtOAc ext. 8.0 0.0 0.0 0.0 n.t.
n-hexane ext. 8.0 7.0 0.0 0.0 n.t.
MeOH ext. 10.0 15.0 0.0 0.0 n.t.
Nostoc sp.
Large scale
EtOAc ext. n.t. n.t. n.t. n.t. n.t.
n-hexane ext. 0.0 0.0 0.0 0.0 n.t.
MeOH ext. 17.0 19.0 12.0 12.0 n.t.
Batch
EtOAc ext. 7.0 6.5 6.5 0.0 n.t.
n-hexane ext. 15.5 8.0 7.5 0.0 n.t.
MeOH ext. 8.0 8.0 7.0 0.0 n.t.
Calothrix
elenkinii
Large scale
EtOAc ext. n.t. n.t. n.t. n.t. n.t.
n-hexane ext. 0.0 0.0 0.0 0.0 n.t.
MeOH ext. 17.0 18.0 8.0 0.0 n.t.
Batch
EtOAc ext. 14.0 12.0 0.0 0.0 n.t.
n-hexane ext. 12.0 0.0 7.0 9.0 n.t.
MeOH ext. 0.0 7.0 7.0 0.0 n.t.
Scytonema
millei
Large scale
EtOAc ext. n.t. n.t. n.t. n.t. n.t.
IZ=Inhibition zone; Ext.=extract; n-hexane ext. and MeOH ext. from dry biomass; EtOAc ext. from culture
medium; n.t.= not test. 1Diameter of inhibition zone (mm) includes Ø disc (6mm) aBacillus subtilic; b Staphylococcus aureus; cEscherichia coli; dPseudomonas aeruginos; eCandida maltosa
Appendix 7: Diameter of inhibition zone of extracts from three cyanobacterial strains in large scale
cultivation in comparison with batch cultivation against test organisms in vitro

185
Appendix 8a: 1H NMR of fraction CJFII-4 of Calothrix javanica

186
Appendix 8b: 1H NMR of fraction CJFII-4 of Calothrix javanica

187
Appendix 9a: 1H NMR of fraction AF6 of Anabaena sp.

188
Appendix 9b: 1H NMR of fraction AF6 of Anabaena sp.

189
Appendix 10a: 1H NMR of fraction F8-3-2 of Lyngbya majuscula

190
Appendix 10b: 1H NMR of fraction F8-3-2 of Lyngbya majuscula

191
Appendix 11a: 1H NMR of fraction F10-3 of Lyngbya majuscula

192
Appendix 11b: 13C NMR of fraction F10-3 of Lyngbya majuscula

193
Appendix 12a: 1H NMR of fraction F10-5 of Lyngbya majuscula

194
Appendix 12b: 1H NMR of fraction F10-5 of Lyngbya majuscula

195
10.00 15.00 20.00 25.00 30.00 35.00 40.00 45.00 50.00 55.00 60.000
100000
200000
300000
400000
500000
600000
700000
800000
900000
1000000
1100000
1200000
1300000
1400000
1500000
1600000
1700000
1800000
1900000
2000000
2100000
2200000
2300000
2400000
2500000
2600000
2700000
2800000
2900000
Time-->
Abundance
TIC: M210411.D
C14
C16
C18
C20
Appendix 13: GC-MS spectrum for the fatty acids of n-hexane extract of Lyngbya majuscula

196
Appendix 14a: 1H NMR data of fraction CJFII-4 of Calothrix javanica compared with fraction FSO3 of Scytonema ocellatum

197
Appendix 14b:
1H NMR data of fraction CJFII-4 of Calothrix javanica compared with fraction FSO3 of Scytonema ocellatum

198
Appendix 14c: MS of methanol extract of Calothrix javanica compared with MS of methanol extract of Scytonema ocellatum